Clinical and Imaging Analysis of Patients with Severe and Critical Coronavirus Disease 2019 with Different Prognosis
-
摘要: 目的:分析不同预后的重型及危重型新冠肺炎患者影像及临床资料,为临床决策提供帮助。方法:收集重型及危重型新冠肺炎患者的临床资料和胸部CT,临床资料包括:血常规、C反应蛋白、降钙素原(PCT)、肝肾功能、D-二聚体(D-Dimer)、心肌酶、B型氨基端利钠肽原(LNTP)、有无基础病史,比较不同预后两组新冠肺炎患者胸部CT影像及各项指标差异,对两组存在显著差异性的相关指标做二元logistic回归分析。结果:入组118例患者,死亡组68例,生存组50例,死亡组年龄大于生存组,死亡组咳痰与纳差症状的比例更高;与生存组比较,死亡组在白细胞计数(WBC)、中性粒细胞绝对值(NEUT)、单核细胞绝对值(MONO)、红细胞计数(RBC)、血红蛋白(HGB)、红细胞比积(HCT)、肾小球滤过率异常、降钙素原(PCT)、D-Dimer、肌酸激酶同工酶(CK-MB)、肌钙蛋白(TNI)、B型氨基端利钠肽原(LNTP)异常的比例更高;相对于生存组,死亡组的WBC、NEUT及百分率、中性粒细胞/淋巴细胞比值(NLR)、红细胞体积分布宽度(RDW-CV)、红细胞体积分布宽度SD(RDW-SD)、PCT、D-Dimer、肌酸激酶(CK)、CK-MB、肌红蛋白(MYO)、TNI、LNTP值明显升高,而淋巴细胞百分率(%LYMPH)、单核细胞百分率(%MONO)、平均红细胞血红蛋白浓度(MCHC)、肾小球滤过率明显减低。年龄、RBC、肾小球滤过率、CK-MB、MYO、LNTP是提示预后结果的主要因素;与生存组比较,死亡组患者新冠病毒感染肺炎的影像无明显差异,但初始胸部CT病变范围较大,多超过50%;生存组肺部CT病变较多位于肺外周及胸膜下,伴随病程死亡组病变多表现为进展或加重。结论:新冠病毒感染患者的年龄、血常规、肝肾功能、心肌功能、血凝状态、炎性反应物指标、肺部病变范围及进展情况是提示疾病严重程度及预后不良的重要因素;年龄、RBC、肾小球滤过率、CK-MB、MYO、LNTP的异常是提示重型及危重型患者致死性结果的主要危险因素;结合临床及实验室检查综合评估,胸部CT检查及随访是不能缺少的判断疾病严重程度及预后的重要评估方法。Abstract: Objective: This study aimed to analyze imaging and clinical data of patients with severe and critical coronavirus disease 2019 (COVID-19) with different prognoses and provide help for clinical decision-making. Method: Clinical data and chest imaging computed tomography (CT) of patients with severe and critical COVID-19 were collected. Clinical data included: blood routine indexes, C-reactive protein, procalcitonin (PCT), the indexes of liver and kidney function, D-Dimer, myocardial enzyme, B-type amino terminal natriuretic peptide (LNTP), and whether there was any underlying medical history. The chest CT images and various indexes of patients with different prognoses of COVID-19 were compared. The relevant indicators with significant differences between the two groups were analyzed using binary logistic regression. Results: A total of 118 patients were enrolled, including 68 in the death group and 50 in the survival group. The age of the death group was longer, and the proportion of sputum and poor tolerance was higher than that of the survival group. Compared with the survival group, in the death group, there was a higher abnormal proportion of leukocyte count (WBC), neutrophil absolute value, monocyte absolute value, red blood cell count (RBC), hemoglobin, erythrocyte ratio, abnormal glomerular filtration rate, PCT, D-Dimer, creatine kinase isoenzyme (CK), troponin (TNI), LNTP. Compared with the survival group, WBC, NEUT and percentage, neutrophil/lymphocyte ratio, erythrocyte volume distribution width, erythrocyte volume distribution width standard deviation, PCT, D-Dimer, CK, CK-MB, myoglobin (MYO), TNI and LNTP were significantly increased in the death group, while the lymphocyte percentage, monocyte percentage, mean RBC hemoglobin concentration (MCHC), and glomerular filtration rate were significantly lower. Compared with the survival group, there was no significant difference in the imaging signs of COVID-19 infection in the death group, but the scope of initial chest CT lesions was larger, with more than 50%. In the survival group, more CT lesions were located in the periphery of the lung and subpleura, while in the death group, more lesions showed progression or aggravation. Age, RBC, glomerular filtration rate, CK-MB, MYO, and LNTP were the main factors that suggested prognostic outcomes. Conclusion: Age, blood routine, liver and kidney function, myocardial function, hemagglutination status, inflammatory reactant index, and lung lesion extent and progression of patients infected with COVID-19 are important factors indicating the severity of the disease and poor prognosis. Abnormal increases in leukocyte and neutrophilic granulocyte, CRP, PCT, D-dimer, and myocardial markers might be the main factors that better predict fatal outcomes in severe and critical patients. Abnormalities in age, RBC, glomerular filtration rate, CK-MB, MYO, and LNTP were the main factors indicating fatal outcomes in severe and critically ill patients. Combined with the comprehensive evaluation of clinical and laboratory examinations, imaging findings and follow-up are indispensable methods to evaluate the severity and prognosis of the disease.
-
Keywords:
- novel coronavirus /
- pneumonia /
- laboratory examination /
- prognosis
-
新型冠状病毒感染(coronavirus disease 2019,COVID-19)是由新型冠状病毒引起的一种急性呼吸道传染性疾病[1]。该病起病急、传播快、普遍易感,由于侵及呼吸道不同部位而临床表现多样,根据临床分型,将该病分为轻型、普通型、重型及危重型[2]。影像学检查对其的诊断价值不可替代,目前,大多数学者仅单纯探讨COVID-19的肺部影像学表现[3-5],而极少有文献报道影像学检查对于COVID-19分型的诊断价值。
本文回顾性分析2022年12月20日至2022年12月31日于我院感染科诊断为COVID-19的134例患者,并根据临床分型进行分组,对于不同分型的COVID-19患者的临床特点及肺部影像学表现进行分析总结,以探讨胸部薄层CT平扫对于COVID-19分型的诊断价值,为临床诊断、治疗提供影像依据。
1. 材料与方法
1.1 一般资料
回顾性收集2022年12月20日至2022年12月31日期间于北京大学第九临床医学院(首都医科大学附属北京世纪坛医院)感染科确诊为COVID-19的134例患者的临床及影像资料。入组标准:符合国家卫健委《新型冠状病毒感染诊疗方案(试行第十版)》[2]中的诊断标准,且具有完整的胸部薄层CT平扫影像资料。
排除标准:不具备完整的临床资料及影像学检查资料的患者,胸部CT无异常的患者。134例新冠感染患者中,男73例(54.5%),女61例(45.5%),年龄26~98岁,平均年龄(69.6±15.0)岁,平均病程5 d,发热126例(94.0%),咳嗽120例(89.6%),肌痛21例(15.7%),咽痛42例(31.3%),胸闷14例(10.4%),腹泻9例(6.7%),纳差3例(2.2%),合并基础病87例(64.9%)。
1.2 检查方法
134例患者均接受胸部CT扫描,CT扫描仪为32排的北京赛诺威盛Insitum-CT 338机型,扫描参数设置:管电压120 kV,管电流150 mAs,螺距1.0。之后进行三维重建,横断面层厚为肺窗1.5 mm和纵隔窗5 mm,矩阵512×512,FOV 380~450;并进行冠状位和矢状位肺窗(1×5 mm)和纵隔窗(5×5 mm)重建。
1.3 图像分析
由两名放射科医师分别进行胸部CT平扫图像阅片,结果不一致时由另一位具有10年以上工作经验的高级医师评定最终阅片结果。
CT主要指标包括:①病变数量:分为单发和多发,多发又分为≤5个、≤10个和>10个;②部位:单肺、单叶、双肺、对称、非叶段;③分布:周围、中央;其中周围分布分为胸膜下和胸膜内,中央分布又分为沿血管束和血管外;④分布优势:上肺为主、下肺为主、周围为主、中央为主、弥漫分布;⑤病变类型:磨玻璃、实变、网格影、蜂窝影、血管束增厚、混合等;⑥病变边缘:模糊、不规则、光整、分叶、毛刺;⑦形态类型:结节、树芽、斑片、大片、束带状、肿块样、混合;⑧其他征象:小气道壁增厚、血管束增厚、晕征、反晕征、铺路石征、支气管充气征、空气潴留征、拱廊征、煎蛋征、胸膜凹陷征、胸膜尾征、分叶征、空泡征、毛刺征、内部索条、胸膜下黑带、胸膜下线、牵拉性支扩、纤维索条。
1.4 统计学分析
采用SPSS 26.0统计软件,对于不同分型的COVID-19患者的临床特点及肺部CT特征进行统计学分析,计量资料应用独立样本t检验,计数资料应用
$\chi^2$ 检验,P<0.05为差异具有统计学意义。2. 结果
2.1 新型冠状病毒感染患者的临床特点
根据临床分型进行分组,非重症组110例,重症组24例。两组之间合并基础病的差异有统计学意义,且重症组合并基础病(83.3%)的发生率高于非重症组(60.9%);两组间合并糖尿病的差异具有统计学意义,且重症组(45.8%)合并糖尿病的发生率高于非重症组(25.5%)。两组间性别、年龄、平均病程及临床症状的差异均无统计学意义(表1)。
表 1 134例新冠病毒感染患者的临床特点Table 1. Clinical characteristics of 134 patients with COVID-19项目 组别 P 非重症组(n=110) 重症组(n=24) 年龄 68.5±15.2 74.3±12.6 0.090 性别 男 58(52.7) 15(62.5) 0.384 女 52(47.3) 9(37.5) 0.384 平均病程/d 5.0 5.0 0.970 临床特征/例 发热 104(94.5) 22(91.7) 0.949 咳嗽 100(90.9) 20(83.3) 0.465 咽痛 35(31.8) 7(29.2) 0.800 胸闷 11(10.0) 3(12.5) 1.000 肌痛 17(15.5) 4(16.7) 1.000 腹泻 8(7.3) 1(4.2) 0.920 纳差 2(1.8) 1(4.2) 0.450 合并基础病/例 67(60.9) 20(83.3) 0.037 基础病类型/例 高血压 42(38.2) 12(50.0) 0.285 糖尿病 28(25.5) 11(45.8) 0.046 冠心病 22(20.0) 9(37.5) 0.065 脑血管病 16(14.5) 3(12.5) 1.000 2.2 不同分型的新型冠状病毒感染患者的CT表现
非重症组和重症组两组之间病变数量、对称性分布、周围为主分布、弥漫分布、边缘模糊(图1~图5)、大片状(图4)、束带状、血管束增厚、铺路石征(图6)、拱廊征(图5)以及煎蛋征(图1)的差异有统计学意义。重症组的病灶数量>10个(图7)、对称性分布(图6)、弥漫分布、大片状、束带状(图8)、血管束增厚、铺路石征、拱廊征的发生率高于非重症组,而非重症组的周围为主分布、边缘模糊以及煎蛋征的发生率高于重症组(表2)。
![]() 图 1 患者,男,非重症组,41岁,发热5 d,伴咽痛、流涕、咳嗽,Tmax 38.2℃,SPO2 98%。右肺背侧胸膜下见不规则煎蛋征(亚实性结节)(黑箭)Figure 1. A 41-year-old male patient in the non-critical group had a fever for 5 days, accompanied by sore throat, running nose, cough, Tmax 38.2℃, and SPO2 98%. Irregular fried egg sign is observed in the right dorsal subpleural area (black arrow)
图 1 患者,男,非重症组,41岁,发热5 d,伴咽痛、流涕、咳嗽,Tmax 38.2℃,SPO2 98%。右肺背侧胸膜下见不规则煎蛋征(亚实性结节)(黑箭)Figure 1. A 41-year-old male patient in the non-critical group had a fever for 5 days, accompanied by sore throat, running nose, cough, Tmax 38.2℃, and SPO2 98%. Irregular fried egg sign is observed in the right dorsal subpleural area (black arrow)![]() 图 3 患者,女,非重症组,74岁,间断发热1周余,伴口干、厌食,Tmax 38.2℃,SPO2 98%。双下肺见不规则斑片状实变及磨玻璃密度影Figure 3. A 74-year-old female patient in the non-critical group had an intermittent fever for more than 1 week, accompanied by dry mouth and anorexia, Tmax 38.2℃, and SPO2 98%. Irregular patchy high-density shadows are observed in both lower lungs
图 3 患者,女,非重症组,74岁,间断发热1周余,伴口干、厌食,Tmax 38.2℃,SPO2 98%。双下肺见不规则斑片状实变及磨玻璃密度影Figure 3. A 74-year-old female patient in the non-critical group had an intermittent fever for more than 1 week, accompanied by dry mouth and anorexia, Tmax 38.2℃, and SPO2 98%. Irregular patchy high-density shadows are observed in both lower lungs![]() 图 4 患者,男,重症组,68岁,发热1周,伴咽痒、咳嗽,Tmax 39.3℃,SPO2 95%。右肺可见大片状磨玻璃密度影,胸膜内分布,可见胸膜下黑线(黑箭)Figure 4. A 68-year-old male patient in the critical group had a fever for 1 week accompanied by an itchy throat and cough, Tmax 39.3℃, and SPO2 95%. A large flake of ground-glass opacity is seen in the right lung, distributed within the pleura, with a black subpleural line(black arrow)
图 4 患者,男,重症组,68岁,发热1周,伴咽痒、咳嗽,Tmax 39.3℃,SPO2 95%。右肺可见大片状磨玻璃密度影,胸膜内分布,可见胸膜下黑线(黑箭)Figure 4. A 68-year-old male patient in the critical group had a fever for 1 week accompanied by an itchy throat and cough, Tmax 39.3℃, and SPO2 95%. A large flake of ground-glass opacity is seen in the right lung, distributed within the pleura, with a black subpleural line(black arrow)![]() 图 5 患者,女,重症组,87岁,咳嗽数天,发热1 h,Tmax 39.0℃,SPO2 89%~90%。右肺见斑片状实变及磨玻璃密度影,边缘略模糊,局部可见拱廊征(黑箭)Figure 5. An 87-year-old female patient in the critical group had a fever for 1 hour with cough for several days, Tmax 39.0℃, and SPO2 89%~90%. Patchy consolidation and ground-glass opacity are seen in the right lung, with slightly blurred edges and arcade-like sign (black arrow)
图 5 患者,女,重症组,87岁,咳嗽数天,发热1 h,Tmax 39.0℃,SPO2 89%~90%。右肺见斑片状实变及磨玻璃密度影,边缘略模糊,局部可见拱廊征(黑箭)Figure 5. An 87-year-old female patient in the critical group had a fever for 1 hour with cough for several days, Tmax 39.0℃, and SPO2 89%~90%. Patchy consolidation and ground-glass opacity are seen in the right lung, with slightly blurred edges and arcade-like sign (black arrow)![]() 图 6 患者,女,重症组,83岁,发热7 d,伴咳嗽、咳痰,Tmax 39.0℃,SPO2 90.1%。双肺周围对称性分布片状磨玻璃密度影,其内可见铺路石征Figure 6. An 83-year-old female patient in the critical group had a fever for 7 days with cough and sputum, Tmax 39.0℃, and SPO2 90.1%. The ground-glass opacities are symmetrically distributed around both lungs, and the paving stone sign can be seen within them
图 6 患者,女,重症组,83岁,发热7 d,伴咳嗽、咳痰,Tmax 39.0℃,SPO2 90.1%。双肺周围对称性分布片状磨玻璃密度影,其内可见铺路石征Figure 6. An 83-year-old female patient in the critical group had a fever for 7 days with cough and sputum, Tmax 39.0℃, and SPO2 90.1%. The ground-glass opacities are symmetrically distributed around both lungs, and the paving stone sign can be seen within them![]() 图 7 患者,女,重症组,74岁,发热13 d,伴腹泻、呕吐、全身酸痛、咳嗽,Tmax 37.4℃。双肺多发实变影,沿支气管血管束分布,边缘清楚Figure 7. A 74-year-old female patient in the critical group had a fever for 13 days, accompanied by diarrhea, emesis, body ache, cough, and Tmax 37.4℃. Multiple consolidations in both lungs are distributed along the bronchial vascular bundle with clear edges表 2 不同分型的新冠病毒感染患者的肺部CT表现Table 2. Imaging findings of different subtypes of patients with COVID-19
图 7 患者,女,重症组,74岁,发热13 d,伴腹泻、呕吐、全身酸痛、咳嗽,Tmax 37.4℃。双肺多发实变影,沿支气管血管束分布,边缘清楚Figure 7. A 74-year-old female patient in the critical group had a fever for 13 days, accompanied by diarrhea, emesis, body ache, cough, and Tmax 37.4℃. Multiple consolidations in both lungs are distributed along the bronchial vascular bundle with clear edges表 2 不同分型的新冠病毒感染患者的肺部CT表现Table 2. Imaging findings of different subtypes of patients with COVID-19项目 参数 组别 P 非重型(n=110) 重型(n=24) 数量 单个 2(1.8) 0(0.0) 1.000 多个 108(95.5) 24(100.0) 1.000 ≤5个 14(12.7) 1(4.2) 0.397 ≤10个 24(21.8) 1(4.2) 0.085 >10个 70(63.6) 22(91.7) 0.007 部位 单肺 17(15.5) 1(4.2) 0.255 单叶 10(9.1) 0(0.0) 0.268 双肺 94(85.5) 23(95.8) 0.296 分布 对称 57(51.8) 19(79.2) 0.014 非叶段 94(85.5) 23(95.8) 0.296 周围 108(98.2) 23(95.8) 0.450 膜下 76(69.1) 21(87.5) 0.068 膜内 104(94.5) 23(95.8) 1.000 中央 95(86.4) 21(87.5) 1.000 血管束 95(86.4) 21(87.5) 1.000 血管外 10(9.1) 4(16.7) 0.465 病变分布优势 上肺为主 14(12.7) 2(8.3) 0.799 下肺为主 50(45.5) 7(29.2) 0.144 周围为主 53(48.2) 5(20.8) 0.014 中央为主 20(18.2) 4(16.7) 1.000 弥漫 38(34.5) 15(62.5) 0.011 病变类型 磨玻璃 102(92.7) 24(100.0) 0.375 实变 50(45.5) 12(50.0) 0.686 网格 87(79.1) 22(91.7) 0.253 蜂窝 10(9.1) 1(4.2) 0.700 混合 100(90.9) 24(100.0) 0.268 病变边缘 模糊 62(56.4) 8(33.3) 0.041 不规则 54(49.1) 7(29.2) 0.076 光整 1(0.9) 0(0.0) 1.000 分叶 5(4.5) 0(0.0) 0.585 毛刺 24(21.8) 3(12.5) 0.453 形态类型 结节 91(82.7) 18(75.0) 0.554 树芽 42(38.2) 5(20.8) 0.107 斑片 89(80.9) 23(95.8) 0.138 大片 57(51.8) 19(79.2) 0.014 束带状 38(34.5) 17(70.8) 0.001 肿块样 0(0.0) 1(4.2) 0.179 混合 97(88.2) 23(95.8) 0.458 征象 小气道壁厚 84(76.4) 15(62.5) 0.161 血管束增厚 44(40.0) 24(100.0) 0.000 晕征 80(72.7) 18(75.0) 0.820 反晕征 39(35.5) 13(54.2) 0.088 铺路石 63(57.3) 19(79.2) 0.046 支气管充气征 78(70.9) 21(87.5) 0.094 空气潴留征 38(34.5) 6(25.0) 0.367 拱廊征 38(34.5) 15(62.5) 0.011 煎蛋征 63(57.3) 8(33.3) 0.033 胸膜凹陷征 21(19.1) 4(16.7) 1.000 胸膜尾征 54(49.1) 8(33.3) 0.161 分叶征 11(10.0) 1(4.2) 0.608 空泡征 62(56.4) 17(70.8) 0.192 毛刺征 50(45.5) 11(45.8) 0.973 内部索条 35(31.8) 4(16.7) 0.139 胸膜下黑带 59(53.6) 19(79.2) 0.022 胸膜下线 29(26.4) 4(16.7) 0.318 牵拉性支扩 61(55.5) 17(70.8) 0.166 纤维索条 75(68.2) 17(70.8) 0.800 3. 讨论
3.1 新型冠状病毒感染的临床特点
COVID-19是一种新型呼吸道传染性疾病,其致病病原体为一种单链RNA病毒SARS-CoV-2,该病有较强的传染性且人群普遍易感[4]。该病发病机制尚不十分清楚,可能是由病毒的S-蛋白与人血管紧张素转化酶Ⅱ相互作用感染人呼吸道上皮细胞所致[6]。其组织病理学包括:肺泡弥漫性损伤,肺泡间隔充血、水肿,单核细胞和淋巴细胞弥漫浸润,Ⅱ型肺泡上皮细胞显著增生并脱落,肺透明膜形成,微血管透明血栓的形成;疾病进展时肺泡腔内渗出实变,肺组织出现灶性出血及出血性梗死,肺泡腔渗出物机化以及肺间质纤维化导致肺泡结构破坏[7-8]。患者通常有流行病学史,临床表现主要为发热、咳嗽、咳痰、咽痛、肌痛、腹泻等[9]。老年人以及合并基础病的患者预后较差。
本研究按照临床分型将新冠患者分为非重症组和重症组,两组之间合并基础病、合并糖尿病的差异有统计学意义,提示合并基础病的患者尤其是合并糖尿病的患者更容易出现重症感染,这与既往研究结果一致[10],可能与机体免疫能力有关,具体原因和机制有待进一步研究。
3.2 不同分型的新型冠状病毒感染的肺部CT表现对比分析
胸部薄层CT平扫对于COVID-19的诊断具有独特优势,其可以显示肺部病变的影像学特征和累及范围,对于COVID-19的诊断以及分型具有指导价值。既往文献[3-5]报道,COVID-19肺部CT早期表现主要为多发斑片状或结节状磨玻璃密度影,双下肺外周背侧分布为主,多靠近胸膜并与胸膜平行,可伴有实变影及小叶间隔增厚,病灶内可见支气管充气征及血管束增粗等表现;随着疾病进展,病灶数量增多、范围增大,逐渐沿支气管血管束从外周向中央扩展,病灶密度增高,磨玻璃、实变或索条影等多种形态病变混合存在,可伴有牵拉性支扩,少数患者可见少量胸腔积液;严重者双肺呈弥漫性病变,实变影为主,部分患者呈“白肺”改变,可伴有支气管扩张、肺结构扭曲及肺不张等改变。
本组研究发现,在COVID-19非重症组与重症组之间,在病灶数量、分布、边缘、形态、血管束增粗表现上有所差异。COVID-19肺部影像学大多表现为多发病灶,本组研究中多发病灶发生率为98.5%,可能是由于新冠病毒为RNA病毒,需要病毒在肺内达到一定数量才可致病,两组之间病灶数量>10个的差异具有统计学意义,提示重症患者的病灶数量多大于10个,这可能与肺内病毒感染数量有关。
两组间周围为主分布、对称分布、弥漫分布以及大片状形态的差异具有统计学意义,非重症组周围为主分布的发生率(48.2%)高于重症组(20.8%),而重症组对称分布(79.2%)、弥漫分布(62.5%)以及大片状形态(79.2%)的发生率高于非重症组(51.8%、34.5% 和51.8%),这可能与疾病的发展过程有关,疾病早期,病变多分布于胸膜下和肺外周1/3,这可能与病毒直径较小,可以很快通过支气管首先到达胸膜下气体交换区域有关[11]。
随着疾病进展,病变数量逐渐增多,病灶逐渐融合呈大片状,向肺门或沿胸膜下蔓延至多个肺叶呈弥漫对称分布。两组间病变边缘模糊的差异具有统计学意义,且非重症组(56.4%)发生率高于重症组(33.3%),可能是由于非重症组病毒数量相对少且病毒直径小,容易通过肺泡孔扩散,引起邻近肺泡腔渗出所致[12],而重症组患者疾病进展较快,肺泡渗出增多,病灶密度增高,边缘相对清晰;两组间束带状形态的差异具有统计学意义,且重症组(70.8%)发生率高于非重症组(34.5%),可能是由于重症组患者疾病进程快,病灶此消彼长,呈现形态不规则、密度不均质、类型混杂性的特点[13],平行于胸膜的部分病灶出现机化收缩而呈现束带状。两组间血管束增粗的差异具有统计学意义,且重症组(100.0%)发生率高于非重症组(40.0%),可能是由于重症组患者血管周围间质水肿更重所致。
既往文献[3-5,14-15]报道,COVID-19的肺部CT表现多伴有晕征、反晕征、铺路石征及支气管充气征等征象,而未有文献报道不同分型的COVID-19患者的影像学特殊征象的差异。铺路石征是指在磨玻璃密度病灶内可见网格影,两组间铺路石征的差异具有统计学意义,且重症组(79.2%)发生率高于非重症组(57.3%),可能是由于疾病早期主要以肺泡壁增厚、肺泡内浆液渗出为主,而间质增厚较少,随着疾病进展肺泡间隔扩张充血、小血管网增多以及小叶间隔间质水肿,从而铺路石征的表现增多[16]。
煎蛋征是指亚实性结节,即中心为实性成分、周围伴磨玻璃密度影的结节灶,两组间煎蛋征的差异具有统计学意义,且非重症组(57.3%)发生率高于重症组(33.3%),可能是由于病变早期结节样病灶相对多见,并且磨玻璃密度结节中心区域肺泡进一步损伤所致,而重症组患者病灶逐渐融合为片状,煎蛋样结节状病灶相对少见;拱廊征是指边缘清晰而弯曲的实变带,与胸膜围成拱形,是机化与纤维化的表现之一[13],两组间拱廊征的差异具有统计学意义,且重症组(62.5%)发生率高于非重症组(34.5%),可能是由于重症组疾病发展呈现更明显的多形性及混杂性,部分病灶内出现纤维化改变,可能表示该处肺组织处于修复状态。
3.3 研究的局限性
本研究的局限性:①未纳入临床实验室指标、治疗方法及患者预后等进行比较;②单纯比较不同分型新冠感染患者的影像学表现,未能进一步探讨影像分型与临床分型的相关性;③本研究以患者首诊 CT表现为主,未能进一步观察不同分型患者肺部病灶的动态演变规律。
综上所述,胸部薄层CT平扫能够明确COVID-19患者肺部异常影像学表现,准确评估病灶数量、分布范围、形态特点,其中病灶数量、分布特点、病灶边缘、形态类型及铺路石征、拱廊征、煎蛋征等特殊征象能够有效提示COVID-19的分型,对于COVID-19的精准诊断、治疗选择及患者预后具有重要意义。
-
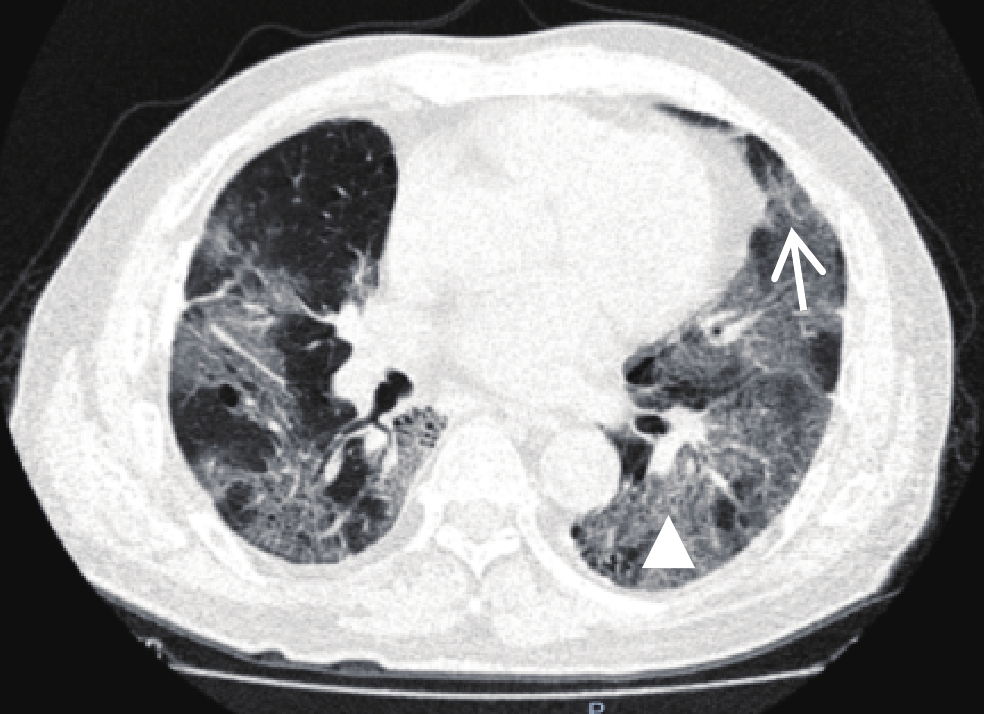
图 1 男性,67岁,死亡患者,肺部CT显示双肺多发磨玻璃影,小叶间隔增厚呈“铺路石征”(三角形),血管穿行于病灶内呈“血管增粗征”(白色箭号)
Figure 1. A 67-year-old male patient (died). A lung CT showed multiple ground glass shadows in both lungs, thickened interlobular septum as a "paving stone sign" (triangle) and blood vessels passing through the lesion as a "thickened vessel sign" (white arrow)
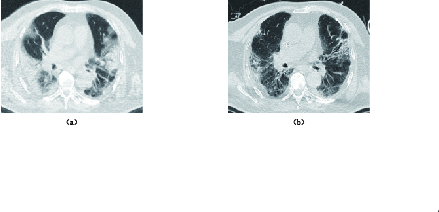
图 2 男性,66岁,死亡患者。行肺部CT平扫检查,显示病变多位于双肺胸膜下,初始病变范围未超过50%,但病变明显进展
Figure 2. A 66-year-old male patient (died). A lung CT scan was performed on day 1, 6, and 15 of admission. The lesions were mostly subpleural in both lungs, and the initial lesions did not exceed 50%, but the lesions progressed significantly
表 1 不同预后两组新型冠状病毒感染者临床特征对比
Table 1 Comparison of clinical features between the two groups of patients with different prognosis
项目 组别 统计检验 死亡组(n=68) 生存组(n=50) $\chi^2 $ P 男性/女性(人数) 46/22 31/18 0.171 0.679 年龄/岁 78.00±12.02 70.04±12.58 -3.416 0.001 症状/例(%) 发热 50(73.5) 40(80.0) 0.667 0.414 高热 11(16.1) 11(22.0) 0.644 0.422 畏寒 5(7.3) 0(0.0) 3.839 0.050 咽痛 7(10.2) 2(4.0) 1.620 0.203 咳嗽 26(38.2) 16(32.0) 0.489 0.485 咳痰 24(35.2) 8(16.0) 5.427 0.020 咳血 2(2.9) 1(2.0) 0.103 0.748 气促 3(4.4) 1(2.0) 0.512 0.474 喘憋 16(23.5) 10(20.0) 0.209 0.648 呼吸不畅 9(13.2) 2(4.0) 2.907 0.088 乏力 18(26.4) 6(12.0) 3.724 0.054 纳差 6(8.8) 0(0.0) 4.648 0.031 胸痛 3(4.4) 0(0.0) 2.263 0.132 胸闷 9(13.2) 3(6.0) 1.651 0.199 基础疾病/例(%) 高血压 43(63.2) 31(62.0) 0.019 0.891 糖尿病 23(33.8) 15(30.0) 0.193 0.660 冠心病 8(11.8) 7(14.0) 0.130 0.719 恶性肿瘤 3(4.4) 1(2.0) 0.512 0.474 免疫抑制(移植术
后、化疗等)4(5.9) 2(4.0) 0.212 0.646 肺心病 1(1.5) 0(0.0) 0.742 0.389 肝病(肝炎,肝硬
化,肝衰竭等)4(5.9) 5(10.0) 0.693 0.405 外科手术史 20(29.4) 19(38.0) 0.077 0.782 表 2 不同预后两组新型冠状病毒感染者实验室指标变化情况比较
Table 2 Comparison of the changes of laboratory indexes in the two groups with different prognoses
项目 组别 统计检验 死亡组(n=68) 生存组(n=50) $\chi^2 $ P WBC升高/例(%) 53(77.9) 18(36.0) 21.552 <0.001 NEUT升高/例(%) 56(82.4) 27(54.0) 11.559 0.003 LYMPH降低/例(%) 47(69.1) 38(76.0) 0.840 0.657 MONO升高/例(%) 25(36.8) 8(16.0) 8.215 0.016 %NEUT升高/例(%) 59(86.8) 37(74.0) 3.813 0.149 %LYMPH减低/例(%) 62(91.2) 44(88.0) 1.435 0.488 %MONO正常/例(%) 39(57.4) 34(68.0) 2.761 0.251 RBC减低/例(%) 57(83.8) 38(56.0) 12.950 0.002 HGB减低/例(%) 57(83.8) 29(58.0) 9.722 0.002 HCT减低/例(%) 61(89.7) 0(0.0) 96.061 <0.001 NLR升高/例(%) 63(92.6) 45(90.0) 0.260 0.610 RDW-SD升高/例(%) 19(27.9) 34(68.0) 12.868 0.002 PLT正常/例(%) 24(35.3) 8(16.0) 7.538 0.053 ALT升高/例(%) 23(33.8) 17(34.0) 0.000 0.984 AST升高/例(%) 39(59.4) 25(50.0) 0.628 0.428 eGFR减低/例(%) 62(91.2) 28(56.0) 19.700 <0.001 SO2(%)≤93%/例(%) 50(75.8) 37(77.1) 5.392 0.056 P/F index≤30 mmHg/例(%) 37(56.1) 36(73.5) 3.676 0.055 CRP升高/例(%) 66(97.1) 44(88.0) 3.741 0.053 PCT升高/例(%) 63(92.6) 27(45.0) 34.667 <0.001 D-Dimer升高/例(%) 56(86.2) 33(68.8) 4.999 0.025 CK升高/例(%) 22(36.7) 6(24.0) 1.282 0.258 CK-MB升高/例(%) 14(23.3) 2(4.7) 6.663 0.010 MYO升高/例(%) 52(86.7) 15(93.8) 0.607 0.436 TNI升高/例(%) 50(83.3) 14(34.1) 25.385 <0.001 LNTP升高/例(%) 60(95.2) 32(76.2) 8.428 0.004 注:WBC为白细胞计数,NEUT为中性粒细胞绝对值,LYMPH为淋巴细胞绝对值,MONO为单核细胞绝对值,%NEUT为中性粒细胞百分率,%LYMPH为淋巴细胞百分率,%MONO为单核细胞百分率,RBC为红细胞计数,HGB为血红蛋白,HCT为红细胞比积,NLR为中性粒细胞/淋巴细胞比值,RDW-SD为红细胞体积分布宽度SD,PLT为血小板计数,ALT为丙氨酸氨基转移酶,AST为天门冬氨酸氨基转移酶,eGFR为肾小球滤过率,SO2为血氧饱和度,P/F index为肺动脉氧分压与吸氧浓度的比值,CRP为C反应蛋白,PCT为降钙素原,D-Dimer为D二聚体,CK为肌酸激酶,CK-MB为肌酸激酶同工酶,MYO为肌红蛋白,TNI为肌钙蛋白,LNTP为B型氨基端利钠肽原。 表 3 不同预后两组新型冠状病毒感染者实验室检查比较
Table 3 Comparison of laboratory indexes between the two groups with different prognoses
项目 组别 统计检验 死亡组(n=68) 生存组(n=50) Z/F P WBC/(×109L) 13.86±6.12 11.56±21.90 -4.670 <0.001 NEUT/(×109/L) 12.23±6.02 7.20±4.22 -4.621 <0.001 LYMPH/(×109/L) 0.912±0.75 3.84±21.36 -0.221 0.825 MONO/(×109/L) 0.57±0.49 0.45±0.31 -0.915 0.360 %NEUT/% 86.10±10.00 79.96±15.02 -2.963 0.003 %LYMPH/% 9.03±9.45 13.55±14.00 -3.154 0.002 %MONO/% 4.30±2.98 5.61±3.28 -2.310 0.021 RBC/(×1012/L) 3.08±1.12 3.76±0.90 2.029 0.157 HGB/(g/L) 94.47±29.12 114.56±28.00 0.308 0.580 HCT/% 29.40±9.04 34.53±7.59 1.382 0.242 NLR 24.34±33.91 11.84±11.97 -3.377 0.001 MCV/FL 97.59±10.09 92.68±7.72 1.581 0.211 MCH/pg 35.80±34.63 30.59±3.02 -0.109 0.913 MCHC/(g/L) 324.90±52.82 330.16±21.05 -3.465 0.001 RDW-CV/% 14.89±2.60 13.55±3.25 -4.524 <0.001 RDW-SD/FL 50.96±9.75 45.03±9.22 -4.316 <0.001 PLT/×109/L 161.88±98.87 209.36±101.18 0.112 0.738 ALT/(U/L) 184.43±626.43 50.72±61.22 -0.249 0.803 AST/(U/L) 276.62±800.04 53.06±47.39 -1.819 0.069 eGFR/(mL/min/1.73 m2) 50.67±39.54 80.89±31.50 -5.065 <0.001 pO2/mmHg 67.18±25.31 58.02±15.48 -1.381 0.167 SO2/% 101.02±92.41 88.58±9.82 -0.878 0.380 P/F index/mmHg 324.65±117.98 282.22±69.21 -1.386 0.166 CRP/(mg/L) 86.37±45.26 59.40±41.20 1.021 0.314 PCT/(ng/mL) 6.63±14.80 2.58±13.30 -5.370 <0.001 D-Dimer/(ug/L) 4281.21±5628.42 2713.70±6835.12 -3.709 <0.001 CK/(U/L) 711.10±1295.57 136.56±292.62 -3.986 <0.001 CK-MB/(ng/mL) 4.58±8.09 1.93±5.60 -4.263 <0.001 MYO/(ng/mL) 1190.55±2064.71 128.31±173.14 -5.701 <0.001 TNI/(ng/mL) 0.89±2.12 0.13±0.31 -5.070 <0.001 LNTP/(pg/mL) 9821.78±21583.97 6934.55±32028.09 -4.840 <0.001 注:WBC为白细胞计数,NEUT为中性粒细胞绝对值,LYMPH为淋巴细胞绝对值,MONO为单核细胞绝对值,%NEUT为中性粒细胞百分率,%LYMPH为淋巴细胞百分率,%MONO为单核细胞百分率,RBC为红细胞计数,HGB为血红蛋白,HCT为红细胞比积,NLR为中性粒细胞/淋巴细胞比值,MCV为平均红细胞体积,MCH为平均红细胞血红蛋白含量,MCHC为平均红细胞血红蛋白浓度,RDW-CV为红细胞体积分布宽度,RDW-SD为红细胞体积分布宽度SD,PLT为血小板计数,ALT为丙氨酸氨基转移酶,AST为天门冬氨酸氨基转移酶,eGFR为肾小球滤过率,pO2为氧分压,SO2为血氧饱和度,P/F index为肺动脉氧分压与吸氧浓度的比值,CRP为C反应蛋白,PCT为降钙素原,D-Dimer为D二聚体,CK为肌酸激酶,CK-MB为肌酸激酶同工酶,MYO为肌红蛋白,TNI为肌钙蛋白,LNTP为B型氨基端利钠肽原。 表 4 临床实验室检查指标的logistic回归分析结果
Table 4 Logistic regression analysis results of clinical laboratory examination indicators
项目 B 标准误 瓦尔德 OR值 OR值95% CI P 年龄/岁 0.276 0.124 4.905 1.317 1.032~1.681 0.027 WBC/(×109/L) 27.876 17.021 2.682 1.277 E+12 0.004~3.935 E+26 0.101 NEUT/(×109/L) -27.394 16.973 2.605 0.000 0.000~354.965 0.107 LYMPH/(×109/L) -30.465 18.544 2.699 0.000 0.000~358.019 0.100 MONO/(×109/L) -10.092 8.903 1.285 0.000 0.000~1568.427 0.257 %NEUT/% 3.076 1.628 3.569 21.681 0.891~527.464 0.059 %LYMPH/% 3.590 1.876 3.662 36.224 0.917~1431.489 0.056 %MONO/% 1.008 0.626 2.596 2.741 0.804~9.346 0.107 RBC/(×1012/L) -34.051 17.049 3.989 0.000 0.000~0.529 0.046 HGB/(g/L) -1.039 0.677 2.355 0.354 0.094~1.334 0.125 HCT/% 6.967 3.696 3.554 1061.421 0.758~1485479.324 0.059 NLR -0.006 0.024 0.066 0.994 0.949~1.041 0.797 MCHC/(g/L) 0.143 0.135 1.126 1.154 0.886~1.502 0.289 RDW-CV/% 1.828 1.820 1.008 6.219 0.175~220.447 0.315 RDW-SD/(FL) -1.173 0.763 2.363 0.309 0.069~1.381 0.124 eGFR/(mL/min/1.73 m2) 0.090 0.042 4.666 1.095 1.008~1.188 0.031 CRP/(mg/L) -0.011 0.019 0.309 0.989 0.953~1.027 0.579 PCT/(ng/mL) -0.344 0.212 2.629 0.709 0.468~1.074 0.105 D-Dimer/(ug/L) 0.000 0.000 1.957 1.000 0.999~1.000 0.162 CK/(U/L) -0.003 0.002 1.900 0.997 0.992~1.001 0.168 CK-MB/(ng/mL) 0.433 0.255 2.878 1.542 0.935~2.543 0.090 MYO/(ng/mL) 0.024 0.012 4.457 1.025 1.002~1.048 0.035 TNI/(ng/mL) 4.129 4.061 1.034 62.119 0.022~177947.798 0.309 LNTP/(pg/mL) 0.000 0.000 4.392 1.000 1.000~1.000 0.036 注:WBC为白细胞计数,NEUT为中性粒细胞绝对值,LYMPH为淋巴细胞绝对值,MONO为单核细胞绝对值,%NEUT为中性粒细胞百分率,%LYMPH为淋巴细胞百分率,%MONO为单核细胞百分率,RBC为红细胞计数,HGB为血红蛋白,HCT为红细胞比积,NLR为中性粒细胞/淋巴细胞比值,MCHC为平均红细胞血红蛋白浓度,RDW-CV为红细胞体积分布宽度,RDW-SD为红细胞体积分布宽度SD,eGFR为肾小球滤过率,CRP为C反应蛋白,PCT为降钙素原,D-Dimer为D二聚体,CK为肌酸激酶,CK-MB为肌酸激酶同工酶,MYO为肌红蛋白,TNI为肌钙蛋白,LNTP为B型氨基端利钠肽原。 表 5 不同预后两组新型冠状病毒肺炎影像特征对比
Table 5 Comparison of imaging features of the two groups with different prognosis of novel coronavirus pneumonia
项目 级别 统计检验 死亡组(n=58) 生存组(n=36) $Z/\chi^2 $ P 发病到CT检查时间/d 9.86±6.50 12.14±6.90 -1.863 0.062 就诊时病变超过>50%/例(%) 33(56.8) 12(33.3) 4.580 0.032 病灶多位于肺外周或胸膜下/例(%) 27(46.6) 26(72.2) 6.340 0.010 磨玻璃影/例(%) 50(86.2) 33(91.7) 0.970 0.325 实变影/例(%) 39(67.2) 24(66.7) 0.114 0.736 铺路石征/例(%) 24(41.4) 18(50.0) 0.788 0.375 血管穿行于病灶内,伴血管增粗/例(%) 32(55.2) 23(63.9) 0.854 0.355 胸膜增厚/例(%) 54(93.1) 32(88.9) 0.181 0.670 淋巴结肿大/例(%) 6(10.3) 1(2.8) 1.790 0.181 胸腔积液/例(%) 24(41.4) 6(16.7) 5.966 0.015 心包积液/例(%) 1(1.7) 1(2.8) 0.127 0.721 病变复查/例 n=33* n=28* 24.820 <0.001 进展/例(%) 27(81.8) 6(21.4) 好转/例(%) 5(15.2) 22(78.6) 变化不大/例(%) 1(3.0) 0(0.0) 病变进展范围超过50%/例(%) 11(33.3) 0(0.0) 3.667 0.056 注:*-死亡组及生存组排除25例和8例没有CT复查图像。 -
[1] CEVIK M, KUPPALLI K, KINDRACHUK J, et al. Virology, transmission, and pathogenesis of SARS-CoV-2[J]. British Medical Journal, 2020, 371: m3862.
[2] IACOBUCCI G. Covid-19: Runny nose, headache, and fatigue are commonest symptoms of omicron, early data show[J]. British Medical Journal, 2021, 375: n3103.
[3] ZHOU F, YU T, DU R, et al. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: A retrospective cohort study[J]. Lancet, 2020, 395(10229): 1054−1062. doi: 10.1016/S0140-6736(20)30566-3
[4] HENRY B M, OLIVEIRA M H S D, BENOIT S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A Meta-analysis[J]. Clinical Chemistry and Laboratory Medicine, 2020, 58(7): 1021−1028. doi: 10.1515/cclm-2020-0369
[5] CAMP J, JONSSON C B. A role for neutrophils in viral respiratory disease[J]. Frontiers in Immunology, 2017, 8: 550. doi: 10.3389/fimmu.2017.00550
[6] ZHANG L, HUANG B, XIA H, et al. Retrospective analysis of clinical features in 134 coronavirus disease 2019 cases[J]. Epidemioogy and Infection, 2020, 148: e199. doi: 10.1017/S0950268820002010
[7] BUJA L M, WOLT D A, ZHAO B, et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus diseas 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities[J]. Cardiovascular Pathology, 2020, 48: 107233. doi: 10.1016/j.carpath.2020.107233
[8] PARACKOVA Z, ZENTSOVA I, BLOOMFFELD M, et al. Disharmonic inffammatory signatures in COVID-19: Augmented neutrophils’ but impaired monocytes’ and dendritic cells’ responsiveness[J]. Cells, 2020, 9(10): 2206. doi: 10.3390/cells9102206
[9] WANG J, LI Q, YIN Y, et al. Excessive neutrophils and neutrophil extracellular traps in COVID-19[J]. Frontiers in Immunology, 2020, 11: 2063. doi: 10.3389/fimmu.2020.02063
[10] Van WOLFSWINKEL M E, VLIEGENTHART-JONGBLOED K, de MENDONÇA MELO M, et al. Predictive value of lymphocytopenia and the neutrophil-lymphocyte count ratio for severe imported malaria[J]. Malaria Journal, 2013, 12: 101. doi: 10.1186/1475-2875-12-101
[11] HUANG W, BERUBE J, McNAMARA M, et al. Lymphocyte subset counts in COVID-19 patients: A Meta-analysis[J]. Cytometry, 2020, 97(8): 772−776. doi: 10.1002/cyto.a.24172
[12] WHERRY E J, KURACHI M. Molecular and cellular insights into T cell exhaustion[J]. Nature Reviews. Immunology, 2015, 15(8): 486−499. doi: 10.1038/nri3862
[13] NG C T, SNELL L M, BROOKS D G, et al. Networking at the level of host immunity: Immune cell interactions during persistent viral infections[J]. Cell Host Microbe, 2013, 13(6): 652−664. doi: 10.1016/j.chom.2013.05.014
[14] CHAN A S, ROUTA A. Use of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in COVID-19[J]. Journal of Clinical Medicine Research, 2020, 12(7): 448−453. doi: 10.14740/jocmr4240
[15] XUE G, GAN X, WU Z, et al. Novel serological biomarkers for inflammation in predicting disease severity in patients with COVID-19[J]. International Immunopharmacology, 2020, 89: (Pt A): 107065.
[16] LU G, WANG J. Dynamic changes in routine blood parameters of a severe COVID-19 case[J]. Clinica Chimica Acta, 2020, 508: 98−102. doi: 10.1016/j.cca.2020.04.034
[17] THOMAS T, STEFANONI D, DZIECIATKOWSKA M, et al. Evidence of structural protein damage and membrane lipid remodeling in red blood cells from COVID-19 patients[J]. Journal of Proteome Research, 2020, 19(11): 4455−4469. doi: 10.1021/acs.jproteome.0c00606
[18] LAZARIAN G, QUINQUENEL A, BELLAL M, et al. Autoimmune haemolytic anaemia associated with COVID-19 infection[J]. British Journal of Haematology, 2020, 190(1): 29−31. doi: 10.1111/bjh.16794
[19] KITCHENS C S. Thrombocytopenia and thrombosis in disseminated intravascular coagulation (DIC)[J]. Hematology, 2009: 240-246.
[20] 朱明超, 朱娅, 郭飞波, 等. 不同预后新型冠状病毒肺炎患者的临床和实验室特征分析[J]. 中华危重病急救医学, 2020,32(12): 1428−1433. doi: 10.3760/cma.j.cn121430-20200824-00590 ZHU M C, ZHU Y, GUO F B, et al. Clinical and laboratory characteristics of 215 cases of coronavirus disease 2019 with different prognosis[J]. Chinese Critical Care Medicine, 2020, 32(12): 1428−1433. (in Chinese). doi: 10.3760/cma.j.cn121430-20200824-00590
[21] 刘玉建, 仲建全, 冯浩, 等. 新型冠状病毒肺炎患者的高分辨率CT影像学特征[J]. 医疗装备, 2022,35(11): 1−4. doi: 10.3969/j.issn.1002-2376.2022.11.001 LIU Y J, ZHONG J Q, FENG H, et al. Imaging characteristics of high resolution CT for patients with corona virus disease 2019[J]. Medical Equipment, 2022, 35(11): 1−4. (in Chinese). doi: 10.3969/j.issn.1002-2376.2022.11.001
[22] PAN F, YE T H, SUN P, et al. Time course of lung changes at chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia[J]. Radiology, 2020, 295(3): 715−721. doi: 10.1148/radiol.2020200370




 下载:
下载: