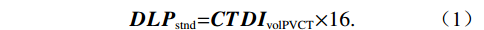One-stop Solution for Coronary CTA and Pulmonary Vein Imaging Based on Extended Field-of-View Reconstruction Technology: Feasibility and Evaluation of Dual Low Radiation and Contrast Agent Doses
-
Abstract:
Purpose: Atrial fibrillation (AF) and coronary artery disease (CAD) share multiple risk factors. Comprehensive imaging evaluation before radiofrequency catheter ablation (RFCA) is beneficial for assessing overall cardiac condition and exploring the underlying causes of AF. This study aimed to evaluate the feasibility of using coronary computed tomography angiography (CCTA)-derived pulmonary vein CT (PVCT) images obtained via extended field-of-view (extDFOV) reconstruction to achieve single-scan imaging for both CCTA and PVCT, with the goal of reducing contrast agent dosage and radiation exposure while maintaining diagnostic image quality. Methods: A total of 44 CAD patients with AF scheduled for RFCA were prospectively enrolled, with 36 meeting the inclusion criteria. All patients underwent both CCTA and PVCT examinations: the experimental group (Group 1) used extDFOV reconstruction from CCTA images to derive PVCT images, while the control group (Group 2) underwent separate helical PVCT scans. Image quality, motion artifacts, signal-to-noise ratio (SNR), contrast-to-noise ratio (CNR), contrast agent dosage, and radiation dose were analyzed and compared between the two groups. Results: CCTA-derived PVCT images (Group 1) demonstrated superior image quality scores (4.44±0.61 vs. 3.97±0.74, P < 0.05) and significantly reduced motion artifacts (EMA: 1.73±0.47 mm vs. 4.55±1.35 mm, P < 0.05) compared to conventional PVCT images (Group 2). The contrast agent dosage was also lower in Group 1 (33.19±3.82 mL vs. 45 mL, P < 0.05). Due to multiphase acquisition in CCTA, the radiation dose in Group 1 was slightly higher than in Group 2 (2.44±0.89 mSv vs. 1.87±0.07 mSv, P < 0.05). Although the CT attenuation values of CCTA-derived PVCT images were lower than those of conventional PVCT, they still provided sufficient enhancement for diagnostic purposes. Conclusion: The use of CCTA-derived PVCT images with extDFOV reconstruction is a feasible and effective method for pre-procedural assessment in CAD patients with AF. This approach significantly reduces contrast agent dosage, minimizes motion artifacts, and optimizes imaging workflow without compromising diagnostic accuracy.
摘要:目的:心房颤动(AF)和冠状动脉疾病(CAD)具有许多共同的发病危险因素,因此在进行射频导管消融术(RFCA)前进行全面的影像学评估有利于对心脏整体状况的了解和对AF病因的探索。本研究旨在评估利用冠状动脉CT血管成像(CCTA)通过扩展视野(extDFOV)重建技术获得衍生的肺静脉CT(PVCT)图像,实现对CCTA和PVCT的单次扫描成像,旨在减少对比剂用量和辐射剂量的同时保持PVCT诊断图像质量的可行性。方法:前瞻性招募了44名计划接受射频消融术的冠状动脉疾病合并心房颤动的患者,最终36名患者符合纳入标准。所有患者均进行了CCTA和PVCT检查:实验组(第1组)使用CCTA图像通过extDFOV扩展视野重建获得的PVCT图像;对照组(第2组)使用常规的螺旋扫描获得的PVCT图像。通过盲法分析并比较两组图像的的主观图像质量、运动伪影、信噪比(SNR)、对比噪声比(CNR)、对比剂用量及辐射剂量。结果:CCTA扩展视野重建的PVCT图像(第1组)表现出更优的主观图像质量评分(4.44±0.61 vs. 3.97±0.74,P < 0.05),并且相比于传统的PVCT(第2组)图像显著减少了运动伪影(EMA:1.73±0.47 mm vs. 4.55±1.35 mm,P < 0.05)。第1组的对比剂用量也低于第2组(33.19±3.82 mL vs. 45 mL,P < 0.05)。辐射剂量方面,由于CCTA采用多期相采集的原因,第1组的辐射剂量略高于第2组(2.44±0.89 mSv vs. 1.87±0.07 mSv,P < 0.05)。CCTA扩展视野重建的PVCT图像(第1组)总体的CT衰减值略低于传统的PVCT(第2组),但仍提供了足够的增强效果以满足诊断需求。结论:利用CCTA衍生的PVCT图像与extDFOV重建技术对CAD合并AF患者的术前评估是可行且有效的。该方法显著减少了对比剂用量,同时减少了运动伪影,优化了影像工作流程而不影响诊断准确性。
-
Atrial fibrillation (AF) is the most prevalent sustained cardiac arrhythmia encountered in clinical, with its incidence increasing significantly with advancing age[1]. AF is associated with a heightened risk of stroke, heart failure, and overall cardiovascular morbidity and mortality, underscoring the importance of early diagnosis and effective management[2]. Coronary artery disease (CAD) is a key contributor to the onset and progression of AF, as it induces atrial ischemia, inflammation, and fibrosis, thereby promoting atrial remodeling and the persistence of AF[3]. Moreover, AF and CAD share multiple common risk factors, including hypertension, diabetes mellitus, obesity, obstructive sleep apnea, smoking, and dyslipidemia[4]. Therefore, comprehensive assessment of both atrial and coronary conditions is essential in the clinical evaluation of AF patients.
Radiofrequency catheter ablation (RFCA) has become the cornerstone treatment for symptomatic, drug-refractory atrial fibrillation[5]. Experts consistently recommend the use of contrast-enhanced computed tomography (CT) imaging of the left atrium (LA) and pulmonary veins (PV) in the perioperative period for thrombus screening and structural assessment. Preprocedural evaluation for RFCA requires detailed visualization of the anatomical structures of the left atrium and pulmonary veins[6]. ECG-gated pulmonary vein imaging facilitates precise measurement of pulmonary vein diameters and allows integration with electroanatomic mapping systems to assist in ablation planning. Additionally, coronary computed tomography angiography (CCTA) provides further insights into the underlying causes of atrial fibrillation and offers a comprehensive assessment of cardiac function and structural integrity[7]. Traditionally, pulmonary vein CT (PVCT) and CCTA have been performed as separate examinations, leading to increased radiation exposure, higher contrast agent consumption, and prolonged scan duration[8-9].
The advent of wide-detector CT technology enables comprehensive cardiac imaging within a single heartbeat. Our study aims to evaluate the feasibility of using a wide-detector CT scanner to simultaneously acquire high-resolution images encompassing the entire heart and major pulmonary vein branches in a single axial scan[10]. By integrating PVCT and coronary CTA into a single acquisition, this approach has the potential to reduce contrast agent dosage and improving the image quality of the pulmonary veins while optimizing the overall imaging workflow.
1. Methods
1.1 Study population
Between June 2022 and December 2022, we prospectively enrolled 44 consecutive patients diagnosed with both coronary artery disease and atrial fibrillation, who were scheduled to undergo radiofrequency ablation. Preoperative evaluation of pulmonary venous anatomy was required for all participants. All patients were in sinus rhythm at the time of imaging, either due to antiarrhythmic treatment or during the interval between episodes of atrial fibrillation, as none had a clinical diagnosis of persistent atrial fibrillation.
Exclusion criteria were as follows: hypersensitivity to iodine-based contrast agents (n=3), severe renal insufficiency (GFR < 30 mL/min, n=3), inability to comply with breath-hold instructions (n=1), and presence of an atrial septal defect (n=1). After applying these criteria, a total of 36 patients were final enrolled in our study.
All 36 patients underwent both coronary computed tomography angiography (CCTA) and helical pulmonary vein computed tomography (PVCT) examinations. The experimental group and the control group were generated according to the acquisition method of PVCT image.
Experimental group (Group 1): PVCT images were reconstructed from the extended display field-of-view (extDFOV) of the axial cardiac scan used for CCTA. In this group, both CCTA and PVCT images were acquired in a single axial scan, the amount of contrast agent and radiation dose were calculated only based on the CCTA imaging. And for control group (Group 2), PVCT images were obtained using helical scan, separate from the CCTA scan. Therefore, the control group patients need to undergo two separate scans to obtain CCTA and PVCT images, each scan requiring separate contrast agent injection and radiation exposure.
To evaluate and compare the quality of the PVCT images between the two groups, a blinded analysis was conducted. The comparisons focused only on the PVCT images and included assessments of subjective image quality, motion artifacts, signal-to-noise ratio (SNR), contrast-to-noise ratio (CNR), contrast agent volume, and radiation dose. The comparison of radiation dose was based on the different scanning protocol in each group and contrast agent volume was calculated based on the actual usage.
This study protocol was reviewed and approved by the Institutional Ethics Committee. All participants provided written informed consent to allow the use of their anonymized imaging data for research purposes.
1.2 CT scanning protocols
All patients underwent both CCTA and PVCT imaging on a 256-row CT scanner with 160 mm detector width (Revolution CT, GE Healthcare). Patients underwent CCTA first followed by PVCT with two contrast medium injections. Both CCTA and PVCT used the bolus-tracking technique to start the scan. CCTA was performed using a prospectively ECG-gated axial scan mode and data acquisition was finished in a single heartbeat. For CCTA, since the 160 mm-wide detector does not cover all the distal pulmonary veins in a single axial scan, CCTA scans were set up to cover the whole heart and included distal pulmonary veins (head-foot direction) as much as possible (Fig. 2). Scan parameters were 256×0.625 mm slices configuration, 280 ms gantry rotation time, 100 kVp tube voltage, SmartmA technique with range of 450~720 mA tube current, and noise index (NI) was set to 11. The region of interest (ROI) for the bolus-tracking was set at the thoracic aorta (Fig. 3). The trigger threshold of 100 HU and a 8.9 s scan delay after triggering were used for starting the diagnostic scan. PVCT was performed using a non-ECG-gated helical scan mode, covering the region from the lung base to the apex in a single breath-hold. For PVCT, the scan parameters were as follows: 100 kVp tube voltage, SmartmA technique with range of 450~720 mA tube current and NI equals 11, 0.28 s gantry rotation time, 80 mm detector collimation width, 0.992:1 helical pitch, and the ROI for the bolus-tracking were placed in the left atrial cavity at the level of two vertebral bodies below the tracheal bifurcation (Fig. 3). The trigger threshold of 140 HU and a 5.9 s scan delay after triggering were used for starting the diagnostic scan.
![]() Figure 2. Schematic diagram of scanning and reconstructionNote: (a) The purple rectangular coverage area represents the scanning range of coronary CTA; (b) The purple rectangular area represents the coverage range of extended field of view reconstruction using coronary CTA scan data; (c) The purple rectangular coverage area represents the range of pulmonary vein spiral scanning.
Figure 2. Schematic diagram of scanning and reconstructionNote: (a) The purple rectangular coverage area represents the scanning range of coronary CTA; (b) The purple rectangular area represents the coverage range of extended field of view reconstruction using coronary CTA scan data; (c) The purple rectangular coverage area represents the range of pulmonary vein spiral scanning.![]() Figure 3. Schematic diagram of region of interest (ROI) placement for the bolus-tracking in coronary computed tomography angiography and pulmonary vein computed tomographyNote: (a) In pulmonary vein computed tomography, ROI for bolus-tracking was placed in left atrial maximal dimension; (b) In coronary computed tomography angiography scan, ROI for bolus-tracking was placed in thoracic aorta.
Figure 3. Schematic diagram of region of interest (ROI) placement for the bolus-tracking in coronary computed tomography angiography and pulmonary vein computed tomographyNote: (a) In pulmonary vein computed tomography, ROI for bolus-tracking was placed in left atrial maximal dimension; (b) In coronary computed tomography angiography scan, ROI for bolus-tracking was placed in thoracic aorta.The contrast media for the examinations were injected through an antecubital vein. In CCTA, patients received body weight-dependent contrast medium volume with different injection rates for 10 s (Iomeron 400 mg iodine/mL; Bracco), followed by the same volume of saline solution at the same injection rate (Table 1). In PVCT, patients received a fixed 45 mL bolus of contrast medium at an injection rate of 4.0 mL/s, followed by a 40 mL flush of saline solution at a rate of 4.0 mL/s.
Table 1. Coronary CTA contrast agent injection protocolbody weight/kg iodine flow rate/(gI/s) injection flow rate/(mL/s) contrast dose/mL saline dose/mL < 50 0.98 2.5 25 25 50~60 1.12 2.8 28 28 60~70 1.26 3.2 32 32 70~80 1.40 3.5 35 35 > 80 1.54 3.9 39 39 1.3 Image reconstruction and analysis
All images were reconstructed using the adaptive statistical iterative reconstruction-V (ASIR-V) algorithm with a reconstruction strength of 50% and a standard reconstruction kernel, with an image slice thickness of 0.625 mm. For coronary CT angiography (CCTA) reconstruction, the SmartPhase technique was used to automatically select the cardiac phase with the least cardiac motion. In addition, the SnapShot Freeze (SSF, GE Healthcare) technique was applied to further reduce residual cardiac motion artifacts. The standard display field of view (DFOV) for CCTA was 25 cm, with a maximum DFOV of 32 cm. Therefore, in this study, an additional set of images (Group 1) was reconstructed using the extended field-of-view (extDFOV) mode with a DFOV of 32 cm. These images were selected from the CCTA acquisition, corresponding to the cardiac phase closest to the isovolumetric relaxation phase (approximately 65%~70% of the RR interval).
It is important to note that while CCTA scanning allows for a maximum reconstruction length of 160 mm, this remains shorter than the coverage achievable with helical pulmonary vein CT (PVCT) scanning. The CCTA-derived PVCT images and the true helical PVCT images were transferred to an independent post-processing workstation (Advantage Workstation, AW Version 4.7, GE Healthcare) for further post-processing and comparative analysis. Axial, sagittal, coronal, multi planar reformat (MPR), and volume rendering (VR) images of LA and PVs were generated (Fig. 4).
![]() Figure 4. Multi planar reformat (MPR) and Volume rendering (VR) images for two GroupsNote: (a) VR image(P-A view) generated using conventional PVCT helical scan data; (b) VR image(P-A view) generated using extDFOV reconstruction in CCTA; (c) VR image(A-P view) generated using conventional PVCT helical scan data; (d) VR image(A-P view) generated using extDFOV reconstruction in CCTA; (e) MPR image(coronal view) generated using conventional PVCT helical scan data; (f) MPR image(coronal view) generated using extDFOV reconstruction in CCTA; (g) MPR image(axial view) generated using conventional PVCT helical scan data; (h) MPR image(axial view) generated using extDFOV reconstruction in CCTA.
Figure 4. Multi planar reformat (MPR) and Volume rendering (VR) images for two GroupsNote: (a) VR image(P-A view) generated using conventional PVCT helical scan data; (b) VR image(P-A view) generated using extDFOV reconstruction in CCTA; (c) VR image(A-P view) generated using conventional PVCT helical scan data; (d) VR image(A-P view) generated using extDFOV reconstruction in CCTA; (e) MPR image(coronal view) generated using conventional PVCT helical scan data; (f) MPR image(coronal view) generated using extDFOV reconstruction in CCTA; (g) MPR image(axial view) generated using conventional PVCT helical scan data; (h) MPR image(axial view) generated using extDFOV reconstruction in CCTA.Two experienced readers (All were attending physicians with more than ten years of experience in imaging diagnosis) independently interpreted all studies for the evaluation of image quality. If there was discordance between the ratings of the two readers, the scoring was re-performed by consensus between the two readers. Image quality was graded on a Likert 5-point scale: 5, no motion artifacts, LA and PVs show clear and homogeneous enhancement; 4, minimal motion artifacts, with clear and homogeneous enhancement in LA and PVs; 3, moderate motion artifacts, with some inhomogeneous enhancement in LA or PVs, but still diagnostic; 2, obvious motion artifacts with inhomogeneous enhancement LA or PVs; 1, severe motion artifacts and inhomogeneous enhancement LA or PVs, not diagnostic. For each patient, the mean objective measurement of CT value was used as indicator for contrast enhancement, expressed as Hounsfield Units (HU), and the corresponding standard deviation (SD) as indicator for image noise, and were measured in a circular 50 mm2 ROI placed on axial images at the level of the LA and at the ostium of each pulmonary vein. Severity of each pulmonary vein and left atrium motion artifacts was assessed using the extend of motion artifact (EMA) measurement. For the left atrium, the EMA value was the extend of the bilateral atrial artifact at the maximum atrial motion artifact slice. For the pulmonary veins, EMA value was the extend of the vessel wall bilaterally in the pulmonary veins at the level of maximum motion artifact (Fig. 5). Additional 100 mm2 ROI was placed on the abdominal fat (fa) with no other structures to calculate the contrast-to-noise ratio (CNR). Image noise was defined using the standard deviation (SD) measurement on the abdominal fat. The CNRs were calculated for the LA (la) as: CNR=[HU (la)-HU (fa)]/SD (fa); and for pulmonary veins (pv) as: CNR=[HU (pv)–HU (fa)]/SD (fa).
![]() Figure 5. Schematic diagram of extend of motion artifact (EMA) distance measurement (The orange short line pointed by the black arrow indicates)Note: (a) Bilateral shadows generated by left atrial wall movement (b) The widest part of the motion artifact caused by cardiac pulsation is measured as the value of EMA
Figure 5. Schematic diagram of extend of motion artifact (EMA) distance measurement (The orange short line pointed by the black arrow indicates)Note: (a) Bilateral shadows generated by left atrial wall movement (b) The widest part of the motion artifact caused by cardiac pulsation is measured as the value of EMA1.4 Radiation does report
The effective dose (ED) of CT was estimated in accordance with the recommendations of the European Working Group for Guidelines on Quality Criteria in CT and the International Commission on Radiological Protection (ICRP). For both coronary CT angiography (CCTA) and pulmonary vein CT (PVCT) scans, the dose-length product (DLP), volume CT dose index (CTDIvol), and scan length of PVCT were recorded. Given the differences in actual scan lengths between the two groups, DLP values for the helical scan group were standardized to a uniform scan length of 16 cm, corresponding to that of the axial scan group. This adjustment was performed using the equation:
$$ \boldsymbol{DLP} _{ \mathrm{stnd}} \mathrm= \boldsymbol{CTDI} _{ \mathrm{volPVCT}} \mathrm{\times 16.} $$ (1) where DLPstnd represents the standardized dose-length product. The effective dose (ED) as recommended for chest CT was subsequently approximated using the equation:
$$ \boldsymbol{ED} \mathrm= \mathit{k} \mathrm{\times DLP}, \;\;\mathit{k} \mathrm{=0.014mSv\cdot mGy}^{ \mathrm{-1}} \cdot \mathrm{cm}^{ \mathrm{-1}} \mathrm{.} $$ (2) 1.5 Statistical analysis
The two imaging groups were compared for differences in image quality score, absolute HU, image noise (SD), SNR, CNR, EMA of atrium and pulmonary veins, and for radiation and contrast doses. Statistical analyses were performed with commercially available SPSS version 26.0 software (SPSS, Chicago, IL, USA). Continuous variables were expressed as mean±SD, and categorical data were expressed in proportions or percentages. All the continuous variables were verified for normality with Shapiro-Wilk test, two-tailed independent t-test was used for comparison of data of continuous variables that conformed to a normal distribution, and non-parametric rank sum test was used for continuous variables that did not conform to a normal distribution. Categorical data was compared by the Mann–Whitney U test. Testing level α=0.05 for a two-sided test.
2. Results
Pulmonary veins and coronary arteries were successfully examined in all patients without any complications.
Study population characteristics are listed in Table 2. Group 1 and Group 2 shared the same patients.
Table 2. Baseline information of study populationCategory Value Age/(year) 60.25±9.63 Sex/(male/female) 18/18 Height/cm 168.92±7.67 Weight/kg 68.42±10.93 Body mass index (BMI) 23.91±3.04 Heart rate (BPM) 73.81±10.18 The objective scores of image quality of LA and PVs from CCTA and PVCT are shown in Table 3. The CCTA-derived PVCT images with extDFOV had a less scan range and DFOV than Group 2 PVCT images, but all images met the diagnostic criteria, and the overall image quality score in Group 1 (4.44±0.61) was better than that in Group 2 (3.97±0.74) with significantly reduced motion artifacts. CCTA-derived PVCT images with extDFOV reconstruction led to an EMA reduction (1.73±0.47) mm vs. (4.55±1.35) mm, P < 0.05). The image noise, SNR, CNR of each PVs and LA in Group 1 were better than Group 2. The CT values in Group 1 were lower than those in Group 2, but both were adequate for diagnosis.
Table 3. Comparison of PVCT image quality between two groupsCategory Group 1(n=36) Group 2 (n=36) P value Test statistics Image Quality Score 4.44±0.61 3.97±0.74 0.006 2.75 LSPV CT Value/HU 527.26±94.03 576.53±155.25 0.03 2.23 LIPV CT Value/HU 495.75±78.23 562.87±128.3 0.02 3.69 RSPV CT Value/HU 522.53±106.22 572.68±144.88 0.03 2.30 RIPV CT Value/HU 482.98±92.54 553.17±129.79 0.02 3.79 LA CT Value/HU 518.71±73.37 618.12±125.68 0.00 5.66 LSPV SD Value 24.65±8.75 45.63±8.03 0.00 11.53 LIPV SD Value 27.12±6.85 48.21±9.29 0.00 11.24 RSPV SD Value 23.19±4.88 43.68±8.29 0.00 12.63 RIPV SD Value 26.81±6.81 49.04±9.86 0.00 14.58 LA SD Value 24.69±5.02 50.27±7.56 0.00 19.31 LSPV SNR Value 22.87±6.03 12.55±2.21 0.00 10.48 LIPV SNR Value 19.38±5.61 11.85±2.66 0.00 7.56 RSPV SNR Value 23.32±6.46 13.16±2.44 0.00 9.21 RIPV SNR Value 18.76±4.61 11.43 ± 2.29 0.00 11.23 LA SNR Value 21.51±3.89 12.39±2.15 0.00 12.02 LSPV CNR Value 27.92±6.85 15.18±2.19 0.00 11.51 LIPV CNR Value 23.96±6.68 14.37±2.92 0.00 8.14 RSPV CNR Value 28.59±7.36 15.92±2.58 0.00 9.80 RIPV CNR Value 23.34±5.33 13.91±2.53 0.00 12.55 LA CNR Value 26.38±4.50 14.76±2.33 0.00 7.25 Extend of Motion Artifact/cm 1.73±0.47 4.55±1.35 0.00 −11.59 Contrast agent dose/mL 33.19±3.82 78.19±3.82 0.00 −7.39 Injection rate/(mL/s) 3.31±0.37 4.00±0.00 0.00 −7.86 CTDIvol 10.91±4.00 8.36±0.30 0.00 3.595 DLP 174.535±64.03 133.73±4.82 0.00 3.595 ED 2.44±0.89 1.87±0.07 0.00 3.595 After completing the entire examination process of coronary CTA and pulmonary vein CTA, radiation dose and contrast dose values of the two groups are listed below. The ED in Group 1 and 2 was (2.44±0.89)mSv and (1.87±0.07)mSv, and DLP was (174.535±64.03) and (133.73±4.82)mGy respectively. Contrast agent does was (33.19±3.82)mL and (78.19±3.82)mL in Group 1 and 2, respectively.
LSPV=Left Superior Pulmonary Vein; LIPV=Left Inferior Pulmonary Vein; RSPV=Right Superior Pulmonary Vein; RIPV=Right Inferior Pulmonary Vein; LA=Left Atrium; SD=Standard Deviation; SNR=Signal To Noise Ratio; CNR=Contrast-To-Noise Ratio; DLP=Dose Length Product; ED=Effective Dose.
3. Discussion
In our study, we investigated the feasibility of using CCTA-derived images with extended DFOV to provide PVCT images to reduce radiation dose and contrast dose for CAD patients with AF who otherwise required separate CCTA and PVCT in routine clinical applications. Our results showed that the CCTA-derived PVCT images with extDFOV reconstruction had better image noise, SNR and CNR than those of the conventional PVCT helical scan images. Regarding the overall scan protocol, Group 1, which utilized CCTA-derived PVCT images, exhibited a lower contrast agent dosage compared to Group 2, which employed conventional PVCT acquisition methods (33.19±3.82 mL vs. 45 mL). However, due to the scan length limitation of 160 mm, CCTA-derived PVCT images did not encompass the distal branches of all pulmonary veins. Notably, the implementation of a single-heartbeat ECG-gated axial scan mode during CCTA significantly mitigated motion artifacts in the PVCT images, thereby enhancing image quality. Owing to the adoption of cardiac imaging protocols in Group 1, a subset of patients underwent multiphase scanning, leading to a slightly higher radiation dose over the standardized 16 cm scan length compared to Group 2 (2.44±0.89 mSv vs. 1.87±0.07 mSv).
For patients with atrial fibrillation scheduled for RFCA, PVCT enables accurate measurement of the pulmonary vein ostial area, detection of morphological variations, and assessment of thrombus within the left atrial appendage[11]. The ECG‐gated PVCT data can be integrated into a three‐dimensional mapping system, playing a crucial role in guiding the planning of the radiofrequency ablation pathway and enhancing surgical precision[12]. Atrial fibrillation and coronary artery disease share similar risk factors and age of onset; approximately 30% of patients with atrial fibrillation also suffer from coronary artery disease. Simultaneous pre‐procedural CCTA not only facilitates evaluation of the general condition of the coronary arteries but also aids in identifying the etiology of atrial fibrillation[13]. In addition, CCTA examination has a certain predictive value for the size of left atrial occluders and surgical outcomes. The evaluation of epicardial adipose tissue using CCTA imaging can also facilitate the investigation of atrial fibrillation etiology from an inflammatory perspective and aid in predicting postoperative efficacy. Some studies suggest that performing CCTA in patients with a history of oral anticoagulant use may also help assess the impact of these medications on coronary artery disease[14-17]. Therefore, it is highly advisable for patients undergoing RFCA evaluation to have both PVCT and CCTA examinations. Given that the peak enhancement times for coronary artery and pulmonary vein CTA are similar, and that ECG‐gated cardiac imaging helps to reduce physiological motion artifacts, the imaging protocol proposed in our study combines an appropriate retrospective reconstruction technique to achieve both CCTA and PVCT imaging with a single contrast injection. The results demonstrate that the images obtained through our extended field-of-view reconstruction method fully meet clinical requirements, and also contribute to a reduction in repeated contrast agent administration during the overall examination process. This, in turn, minimizes the potential risk of contrast-induced nephropathy and simplifies the examination workflow[18]. Owing to the need to accommodate the imaging requirements of the coronary arteries, the examination protocol in this study may involve either a single prolonged phase acquisition or multiple phase acquisitions at the same anatomical location during the image capture process. After standard normalization, the resulting radiation dose is slightly higher than that of the spiral scanning group. However, considering the use of ECG‐gated pulmonary vein imaging technology and the simultaneous achievement of the coronary artery imaging objectives, this increase in radiation dose is entirely justified and acceptable.
This study demonstrates that reconstructing pulmonary vein images from CCTA can significantly reduce contrast agent usage and eliminate the need for repeat scanning without compromising CT image quality. The results indicate that CCTA-derived PVCT images reconstructed using the extended field-of-view (extDFOV) technique achieved higher image quality scores and lower motion artifacts (enabled by ECG gating) compared to conventional PVCT images, while substantially reducing contrast agent consumption. In traditional non-gated helical scans, increased motion artifacts can hinder accurate anatomical assessment, making them unsuitable for navigation during RFCA. Moreover, extDFOV-reconstructed CCTA images exhibited superior signal-to-noise ratio (SNR), contrast-to-noise ratio (CNR), and standard deviation (SD) values. Although the CT attenuation values of pulmonary veins in CCTA-derived PVCT images were lower than those in conventional helical PVCT images, the enhancement was sufficient for diagnostic purposes. The lower attenuation values may be attributed to slight differences in the optimal imaging phase for CCTA and PVCT, as well as the significantly lower contrast agent volume used in CCTA compared to PVCT.
However, this study has several limitations. First, the proposed scanning method has a limited scan field-of-view (SFOV, Cardiac Small) and an extended field-of-view (DFOV, 32 cm). Nevertheless, based on our observations and previous literature, a DFOV of 32 cm is sufficient to encompass the entire lung field without affecting the visualization of pulmonary vein structures. Second, the scan coverage differed between the two groups, with Group 2 having a slightly longer scan range than Group 1. This discrepancy may impact the visualization of distal pulmonary vein branches and the comparison of radiation dose. The scan range in Group 1 may not fully capture the distal branches of the superior pulmonary veins, leading to partial truncation of these branches in volume-rendered (VR) or multiplanar reconstruction (MPR) images. However, in the preprocedural evaluation of RFCA patients, the primary objective of pulmonary vein imaging is to visualize the anatomy of the pulmonary vein ostia and assess the presence of left atrial appendage thrombus[19]. The key areas for intraoperative navigation during RFCA are mostly concentrated at the junction of the pulmonary vein ostia and the left atrium. In these applications, visualization of the distal pulmonary vein branches is not required, aligning with the observers evaluation of image usability in this study.
Furthermore, differences in scan length affect radiation dose comparisons. In this study, the radiation dose in Group 1 was slightly higher than the standardized dose for the same scan length in Group 2. This discrepancy is likely due to the use of ECG-gated imaging, which involved multi-phase acquisitions, leading to partial re-exposure of certain volume regions. Additionally, the radiation dose in the normalized Group 2 measurements did not account for the overscan portion of the helical acquisition. The helical scan mode for pulmonary vein imaging is generally suitable for basic anatomical assessment; however, it is not appropriate for intraoperative navigation during RFCA. During intraoperative navigation for RFCA, pulmonary vein imaging data are typically fused with electrophysiological mapping data, which generally covers only a small region centered around the left atrium. Therefore, comprehensive PVCT scanning is not essential, and direct integration of electrophysiological mapping data could further reduce radiation dose. Overall, given that the proposed protocol achieves clinical objectives in a single scan, it still has the potential to minimize the actual radiation dose received by patients.
Finally, this study did not include comparisons with other vascular imaging modalities, such as magnetic resonance angiography (MRA). As a feasibility study, we also did not collect post-ablation outcomes to assess treatment efficacy.
4. Conclusion
Our study demonstrates that in CAD patients with AF, the use of CCTA-derived PVCT images allows to achieve LA and PVs CT imaging is completely feasible with and significantly reduced contrast agent doses, at the same time improved image quality with less motion artifacts compared to the conventional combined CCTA and PVCT.
-
Figure 2. Schematic diagram of scanning and reconstruction
Note: (a) The purple rectangular coverage area represents the scanning range of coronary CTA; (b) The purple rectangular area represents the coverage range of extended field of view reconstruction using coronary CTA scan data; (c) The purple rectangular coverage area represents the range of pulmonary vein spiral scanning.
Figure 3. Schematic diagram of region of interest (ROI) placement for the bolus-tracking in coronary computed tomography angiography and pulmonary vein computed tomography
Note: (a) In pulmonary vein computed tomography, ROI for bolus-tracking was placed in left atrial maximal dimension; (b) In coronary computed tomography angiography scan, ROI for bolus-tracking was placed in thoracic aorta.
Figure 4. Multi planar reformat (MPR) and Volume rendering (VR) images for two Groups
Note: (a) VR image(P-A view) generated using conventional PVCT helical scan data; (b) VR image(P-A view) generated using extDFOV reconstruction in CCTA; (c) VR image(A-P view) generated using conventional PVCT helical scan data; (d) VR image(A-P view) generated using extDFOV reconstruction in CCTA; (e) MPR image(coronal view) generated using conventional PVCT helical scan data; (f) MPR image(coronal view) generated using extDFOV reconstruction in CCTA; (g) MPR image(axial view) generated using conventional PVCT helical scan data; (h) MPR image(axial view) generated using extDFOV reconstruction in CCTA.
Figure 5. Schematic diagram of extend of motion artifact (EMA) distance measurement (The orange short line pointed by the black arrow indicates)
Note: (a) Bilateral shadows generated by left atrial wall movement (b) The widest part of the motion artifact caused by cardiac pulsation is measured as the value of EMA
Table 1 Coronary CTA contrast agent injection protocol
body weight/kg iodine flow rate/(gI/s) injection flow rate/(mL/s) contrast dose/mL saline dose/mL < 50 0.98 2.5 25 25 50~60 1.12 2.8 28 28 60~70 1.26 3.2 32 32 70~80 1.40 3.5 35 35 > 80 1.54 3.9 39 39 Table 2 Baseline information of study population
Category Value Age/(year) 60.25±9.63 Sex/(male/female) 18/18 Height/cm 168.92±7.67 Weight/kg 68.42±10.93 Body mass index (BMI) 23.91±3.04 Heart rate (BPM) 73.81±10.18 Table 3 Comparison of PVCT image quality between two groups
Category Group 1(n=36) Group 2 (n=36) P value Test statistics Image Quality Score 4.44±0.61 3.97±0.74 0.006 2.75 LSPV CT Value/HU 527.26±94.03 576.53±155.25 0.03 2.23 LIPV CT Value/HU 495.75±78.23 562.87±128.3 0.02 3.69 RSPV CT Value/HU 522.53±106.22 572.68±144.88 0.03 2.30 RIPV CT Value/HU 482.98±92.54 553.17±129.79 0.02 3.79 LA CT Value/HU 518.71±73.37 618.12±125.68 0.00 5.66 LSPV SD Value 24.65±8.75 45.63±8.03 0.00 11.53 LIPV SD Value 27.12±6.85 48.21±9.29 0.00 11.24 RSPV SD Value 23.19±4.88 43.68±8.29 0.00 12.63 RIPV SD Value 26.81±6.81 49.04±9.86 0.00 14.58 LA SD Value 24.69±5.02 50.27±7.56 0.00 19.31 LSPV SNR Value 22.87±6.03 12.55±2.21 0.00 10.48 LIPV SNR Value 19.38±5.61 11.85±2.66 0.00 7.56 RSPV SNR Value 23.32±6.46 13.16±2.44 0.00 9.21 RIPV SNR Value 18.76±4.61 11.43 ± 2.29 0.00 11.23 LA SNR Value 21.51±3.89 12.39±2.15 0.00 12.02 LSPV CNR Value 27.92±6.85 15.18±2.19 0.00 11.51 LIPV CNR Value 23.96±6.68 14.37±2.92 0.00 8.14 RSPV CNR Value 28.59±7.36 15.92±2.58 0.00 9.80 RIPV CNR Value 23.34±5.33 13.91±2.53 0.00 12.55 LA CNR Value 26.38±4.50 14.76±2.33 0.00 7.25 Extend of Motion Artifact/cm 1.73±0.47 4.55±1.35 0.00 −11.59 Contrast agent dose/mL 33.19±3.82 78.19±3.82 0.00 −7.39 Injection rate/(mL/s) 3.31±0.37 4.00±0.00 0.00 −7.86 CTDIvol 10.91±4.00 8.36±0.30 0.00 3.595 DLP 174.535±64.03 133.73±4.82 0.00 3.595 ED 2.44±0.89 1.87±0.07 0.00 3.595 -
[1] MICHNIEWICZ E, MLODAWSKA E, LOPATOWSKA P, et al. Patients with atrial fibrillation and coronary artery disease-Double trouble[J]. Advances in Medical Sciences, 2018, 63(1): 30-35. DOI: 10.1016/j.advms.2017.06.005.
[2] LIP G Y H, PROIETTI M, POTPARA T, et al. Atrial fibrillation and stroke prevention: 25 years of research at EP Europace journal[J]. Europace: European pacing, arrhythmias, and cardiac electrophysiology: Journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology, 2023, 25(9): euad226. DOI: 10.1093/europace/euad226.
[3] MEKHAEL M, MARROUCHE N, HAJJAR A H E, et al. The relationship between atrial fibrillation and coronary artery disease: Understanding common denominators[J]. Trends in Cardiovascular Medicine, 2024, 34(2): 91-98. DOI: 10.1016/j.tcm.2022.09.006.
[4] GLADDING P A, LEGGET M, FATKIN D, et al. Polygenic risk scores in coronary artery disease and atrial fibrillation[J]. Heart, Lung and Circulation, 2020, 29(4): 634-640. DOI: 10.1016/j.hlc.2019.12.004.
[5] BERMAN A E, KABIRI M, WEI T, et al. Economic and health value of delaying atrial fibrillation progression using radiofrequency catheter ablation[J]. Circulation: Arrhythmia and Electrophysiology, 2023, 16(4): e011237. DOI: 10.1161/CIRCEP.122.011237.
[6] PASTEUR-ROUSSEAU A, SEBAG F. Cardiac CT-scan: Utility for the management of chest pain, cardiovascular screening and before atrial fibrillation ablation procedure[J]. Annales de Cardiologie et d'Angeiologie, 2020, 69(5): 276-288. DOI: 10.1016/j.ancard.2020.09.028.
[7] STOJANOVSKA J. Enhancing epicardial fat at cardiac CT as foe in atrial fibrillation[J]. Radiology, 2022, 305(1): 66-67. DOI: 10.1148/radiol.221022.
[8] GUO T, DONG Y, YU S. The comparison of the diagnostic value of left atrial pulmonary vein single-phase and dual-phase enhanced CT scanning for left atrial appendage thrombosis and SEC in patients with atrial fibrillation[J]. Computational and Mathematical Methods in Medicine, 2022, 2022: 8679511. DOI: 10.1155/2022/8679511.
[9] IZOE Y, NAGAO M, TOKAI M, et al. Radiation dose for 320-row dose-modulated dynamic coronary CT angiography[J]. Journal of Applied Clinical Medical Physics, 2021, 22(9): 307-312. DOI: 10.1002/acm2.13390.
[10] JIN L, GAO P, WANG K, et al. Intraindividual evaluation of effects of image filter function on image quality in coronary computed tomography angiography[J]. Frontiers in Cardiovascular Medicine, 2022, 9: 840735. DOI: 10.3389/fcvm.2022.840735.
[11] TERES C, SOTO-IGLESIAS D, PENELA D, et al. Personalized paroxysmal atrial fibrillation ablation by tailoring ablation index to the left atrial wall thickness: The 'Ablate by-LAW' single-centre study-a pilot study[J]. Europace: European pacing, arrhythmias, and cardiac electrophysiology: Journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology, 2022, 24(3): 390-399. DOI: 10.1093/europace/euab216.
[12] SCHWARTZ A L, CHORIN E, MANN T, et al. Reconstruction of the left atrium for atrial fibrillation ablation using the machine learning CARTO 3m-FAM software[J]. Journal of Interventional Cardiac Electrophysiology: An International Journal of Arrhythmias and Pacing. 2022, 64(1): 39-47. DOI: 10.1007/s10840-021-01045-4.
[13] CAPPELLO I A, PANNONE L, DELLA ROCCA D G, et al. Coronary artery disease in atrial fibrillation ablation: Impact on arrhythmic outcomes[J]. Europace: European pacing, arrhythmias, and cardiac electrophysiology: Journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology, 2023, 25(12): euad328. DOI: 10.1093/europace/euad328.
[14] BORDI LL, BENEDEK T, KOVÁCS I, et al. Association of atrial fibrillation recurrence with right coronary atherosclerosis and increased left arterial epicardial fat following catheter ablation-results of a multimodality study[J]. Life (Basel), 2023, 13(9): 1891. DOI: 10.3390/life13091891.
[15] WEST H W, SIDDIQUE M, WILLIAMS M C, et al. Deep-learning for epicardial adipose tissue assessment with computed tomography: implications for cardiovascular risk prediction[J]. Cardiovascular imaging Journal of the American College of Cardiology cardiovascular imaging, 2023, 16(6): 800-816. DOI: 10.1016/j.jcmg.2022.11.018.
[16] AHN J, LEE Y S, LEE W, et al. Randomized comparison of progression of atherosclerotic plaques and calcification of coronary artery in atrial fibrillation patients treated with edoxaban versus warfarin (The REPRESENT-AF trial)[J]. The American Journal of Cardiology, 2024, 229: 56-62. DOI: 10.1016/j.amjcard.2024.08.002.
[17] GOITEIN O, FINK N, HAY I, et al. Cardiac CT Angiography (CCTA) predicts left atrial appendage occluder device size and procedure outcome[J]. The American Journal of Cardiology, 2017, 33(5): 739-747. DOI: 10.1007/s10554-016-1050-6.
[18] CHANDIRAMANI R, CAO D, NICOLAS J, et al. Contrast-induced acute kidney injury[J]. Cardiovascular Intervention and Therapeutics, 2020, 35(3): 209-217. DOI: 10.1007/s12928-020-00660-8.
[19] SHI L, WANG C, CHEN H, et al. Ventricular arrhythmias originating from the basal septum of the ventricle: Clinical and electrophysiological characteristics and a systematic ablation approach[J]. Frontiers in Cardiovascular Medicine, 2022, 9: 879381. DOI: 10.3389/fcvm.2022.879381.
-
期刊类型引用(1)
1. 吴凡,刘进,张意,陈阳,陆志凯. 面向CT成像的深度重建算法研究进展. 中国体视学与图像分析. 2022(04): 387-404 .  百度学术
百度学术
其他类型引用(0)




 下载:
下载: