Feasibility of opportunistic osteoporosis screening using an artificial intelligence-based bone density measurement on chest CT scans
-
摘要:
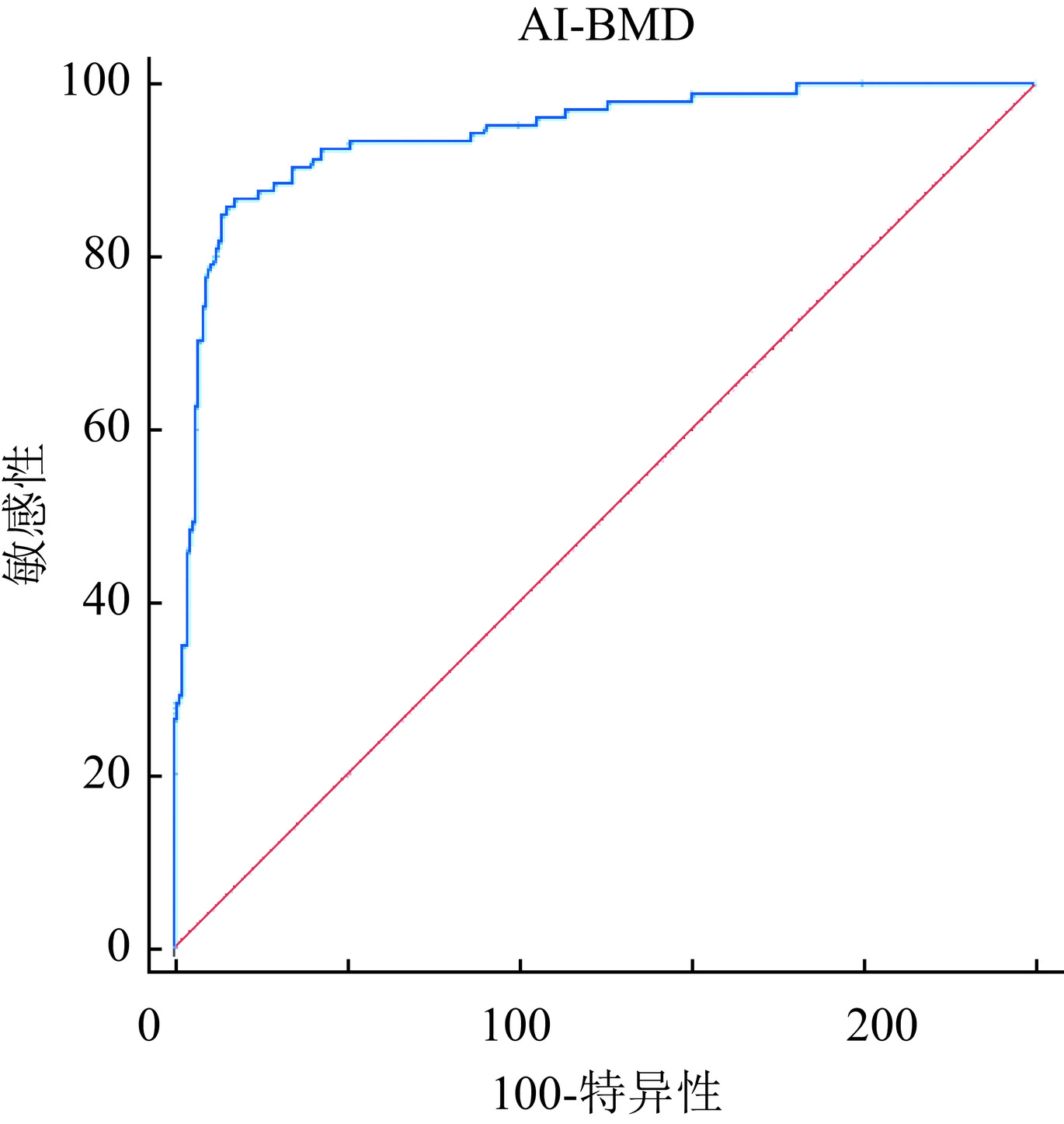
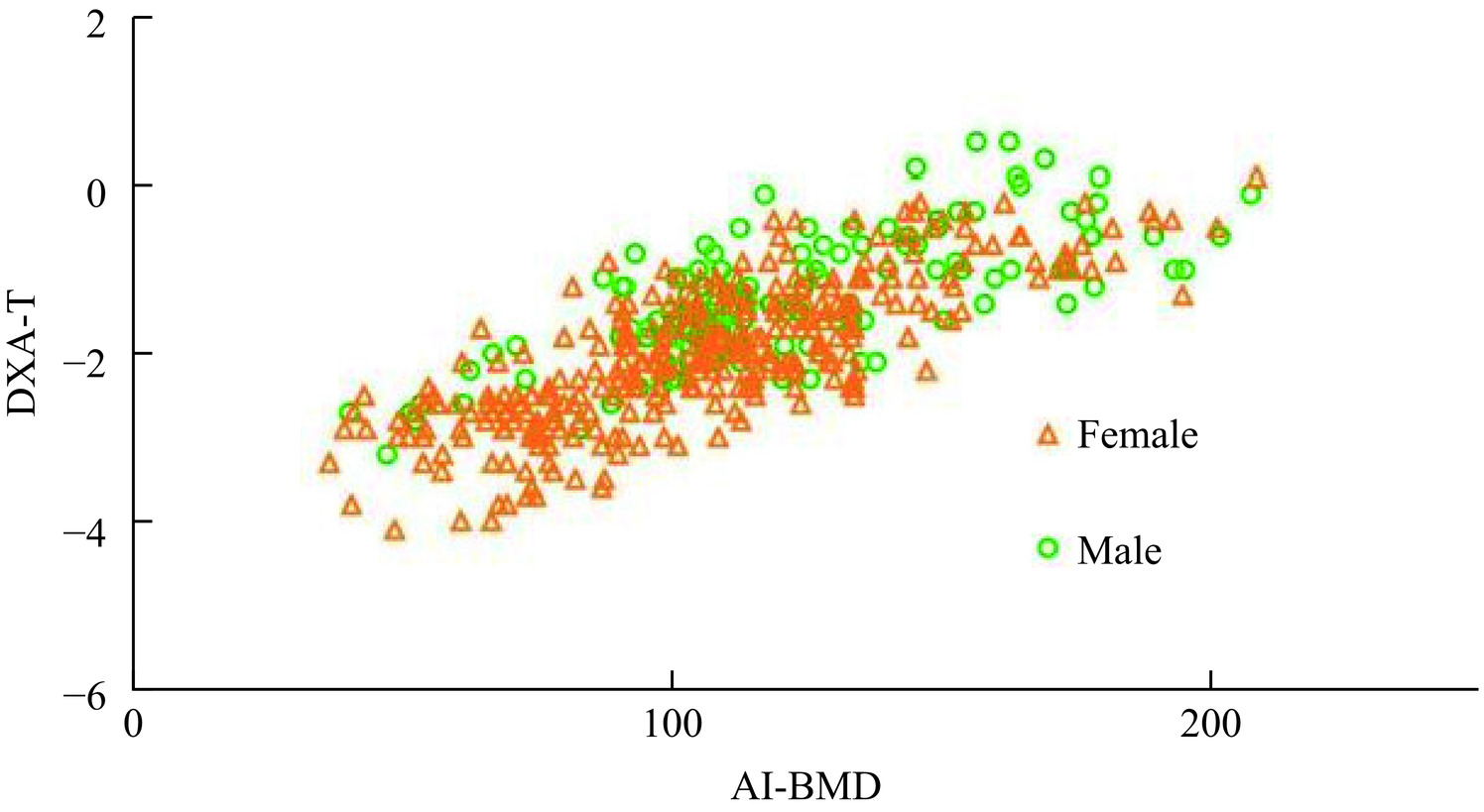
探讨基于胸部CT的人工智能(AI)骨密度测量系统机会性筛查骨质疏松症的可行性。回顾性分析于我科2023年8月至2024年7月同时行双光能X线吸收测定法(DXA)和胸部CT的462名患者的资料,其中绝经后的女性317例、50岁以上的男性145例。比较两种方法测量骨密度之间的差异;以DXA测量的T值为参考标准,分析基于胸部CT的AI系统与DXA测量结果的一致性和相关性。绝经后女性和50岁以上男性的身高、体重、DXA T值及AI BMD有统计学差异;AI测量的BMD与DXA T值之间的相关系数为0.767;二者的κ值为0.697;AI诊断骨质疏松的ROC曲线下面积为0.941(95%CI 0.914~0.968),敏感性85.71%,特异性93.84%。AI骨密度测量系统与DXA测定骨密度具有高度相关性及良好一致性,可以帮助机会性筛查骨质疏松症。
Abstract:This study explores the feasibility of opportunistic osteoporosis screening using an artificial intelligence (AI)-based bone mineral density (BMD) measurement system on chest computed tomography (CT) scans. A retrospective analysis was conducted on 462 patients who underwent both dual-energy X-ray absorptiometry (DXA) and chest CT in our department between August 2023 and July 2024. The cohort included 317 postmenopausal women and 145 men aged > 50 years. BMD measurements from the AI system and DXA were compared. Using the T-value measured by DXA as the reference standard, the consistency and correlation between AI-based and DXA-measured BMD were analyzed. Significant differences in height, weight, DXA T-value, and AI-derived BMD were observed between men aged > 50 years and postmenopausal women. The AI-derived BMD showed a correlation coefficient of 0.767 with DXA T-values and a κ value of 0.697. The area under the ROC curve for AI-based diagnosis of osteoporosis was 0.941(95% CI 0.914–0.968), with a sensitivity of 85.71% and a specificity of 93.84%. The AI-based BMD measurement system demonstrates strong correlation and good agreement with DXA, supporting its feasibility for opportunistic osteoporosis screening.
-
Keywords:
- CT /
- Artificial intelligence /
- Bone density /
- osteoporosis
-
-
表 1 462名受试者基本资料
Table 1 Demographic data from 462 subjects
变量 全部(n=462) 女性(n=317) 男性(n=145) P值 年龄(岁) 64.94±8.547 64.99±8.221 64.81±9.249 0.837 身高(cm) 162.98±7.278 159.35±4.855 170.90±5.065 <0.001 体重(kg) 65.91±11.065 62.52±9.609 73.34±10.412 <0.001 BMI(kg/m2) 24.75±3.385 24.60±3.492 25.08±3.125 0.160 DXA T值 −1.79±0.855 −1.98±0.839 −1.36±0.726 <0.001 AI BMD(mg/cm3) 111.33±33.522 106.66±32.886 121.53±32.732 <0.001 表 2 DXA和AI诊断类型的分布
Table 2 Distributions of DXA and AI diagnostic types
AI 骨量正常 骨量减少 骨质疏松 总计 DXA 骨量正常 67(14.5%) 23(5.0%)a 1(0.2%)b 91(19.7%) 骨量减少 21(4.5%)a 225(48.7%) 20(4.3%)a 266(57.6%) 骨质疏松 0(0%)b 16(3.5%)a 89(19.3%) 105(22.7%) 总计 88(19.0%) 264(57.1%) 110(23.8%) 462(100%) 注:黑体字为一致;a为轻度不一致:一种方法显示骨密度正常,而另一种则显示骨量减少或一种方法显示骨量减少,而另一种则显示骨质疏松;b为重度不一致:一种方法显示骨密度正常,而另一种则显示骨质疏松。 -
[1] LIU F, ZHU H, MA J, et al. Performance of iCare quantitative computed tomography in bone mineral density assessment of the hip and vertebral bodies in European spine phantom[J]. Journal of Orthopaedic Surgery and Research, 2023, 18(1): 777. DOI: 10.1186/s13018-023-04174-w.
[2] CUI Z, MENG X, FENG H, et al. Estimation and projection about the standardized prevalence of osteoporosis in mainland China[J]. Archives of Osteoporosis, 2019, 15(1): 2. DOI: 10.1007/s11657-019-0670-6.
[3] SI L, WINZENBERG T M, JIANG Q, et al. Projection of osteoporosis-related fractures and costs in China: 2010-2050[J]. Osteoporosis International, 2015, 26(7): 1929-1937. DOI: 10.1007/s00198-015-3093-2.
[4] DE MARGERIE-MELLON C, CHASSAGNON G. Artificial intelligence: A critical review of applications for lung nodule and lung cancer[J]. Diagnostic and Interventional Imaging, 2023, 104(1): 11-17. DOI: 10.1016/j.diii.2022.11.007.
[5] 孙安, 樊荣荣, 孙瑶, 等. CT重组算法对低剂量胸部CT筛查冠状动脉钙化积分准确性影响研究[J]. 临床放射学杂志, 2022, 41(5): 881-885. DOI: 10.13437/j.cnki.jcr.2022.05.025. SUN A, FAN R, SUN Y, et al. The effect of CT reconstruction kernel on the accuracy of low-dose chest CT screening for coronary artery calcification scores[J]. Journal of Clinical Radiology, 2022, 41(5): 881-885. DOI: 10.13437/j.cnki.jcr.2022.05.025.
[6] 赵宇, 张晓岚, 郑超, 等. 基于低剂量胸部CT深度学习模型自动测量骨密度研究[J]. 放射学实践, 2024, 39(2): 262-266. DOI: 10.13609/j.cnki.1000-0313.2024.02.019. ZHAO Y, ZHANG X L, ZHENG C, et al. Bone densitometry measurement based on low-dose chest CT with deep learning model[J]. Radiol Practice, 2024, 39(2): 262-266. DOI: 10.13609/j.cnki.1000-0313.2024.02.019.
[7] 赵君禄, 刘斋, 韩康, 等. 基于定量CT的机会性骨质疏松诊断: 2种定量CT骨密度测量软件临床应用比较分析[J]. 河北医科大学学报, 2024, 45(1): 17-23. DOI: 10.3969/j.issn.1007-3205.2024.01.005. ZHAO J L, LIU Z, HAN K, et al. Computed tomography—based opportunistic osteoporosis diagnosis: A comparison of clinical applications of two quantitative CT softwares[J]. Journal of Hebei Medical University, 2024, 45(1): 17-23. DOI: 10.3969/j.issn.1007-3205.2024.01.005.
[8] SIRIS E S, ADLER R, BILEZIKIAN J, et al. The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group[J]. Osteoporosis International, 2014, 25(5): 1439-1443. DOI: 10.1007/s00198-014-2655-z.
[9] BUDOFF M J, HAMIRANI Y S, GAO Y L, et al. Measurement of thoracic bone mineral density with quantitative CT[J]. Radiology, 2010, 257(2): 434-440. DOI: 10.1148/radiol.10100132.
[10] KENDRICK J, FRANCIS R J, HASSAN G M, et al. Prognostic utility of RECIP 1.0 with manual and AI-based segmentations in biochemically recurrent prostate cancer from [(68)Ga]Ga-PSMA-11 PET images[J]. European Journal of Nuclear Medicine and Molecular Imaging, 2023, 50(13): 4077-4086. DOI: 10.1007/s00259-023-06382-2.
[11] GERETY E L, HOPPER M A, BEARCROFT P W. The reliability of measuring the density of the L1 vertebral body on CT imaging as a predictor of bone mineral density[J]. Clinical Radiology, 2017, 72(2): 177-179. DOI: 10.1016/j.crad.2016.09.022.
[12] YAN L, WANG X, YU T, et al. Characteristics of the gut microbiota and serum metabolites in postmenopausal women with reduced bone mineral density[J]. Frontiers in Cellular and Infection Microbiology, 2024, 14: 1367325. DOI: 10.3389/fcimb.2024.1367325.
[13] WU X, ZHANG M. Effects of androgen and progestin on the proliferation and differentiation of osteoblasts[J]. Experimental and Therapeutic Medicine, 2018, 16(6): 4722-4728. DOI: 10.3892/etm.2018.6772.
[14] KIM Y W, KIM J H, YOON S H, et al. Vertebral bone attenuation on low-dose chest CT: Quantitative volumetric analysis for bone fragility assessment[J]. Osteoporosis International, 2017, 28(1): 329-338. DOI: 10.1007/s00198-016-3724-2.
[15] SAVAGE R H, van ASSEN M, MARTIN S S, et al. Utilizing Artificial Intelligence to Determine Bone Mineral Density Via Chest Computed Tomography[J]. Journal of Thoracic Imaging, 2020, 35 Suppl 1: S35-S39. DOI: 10.1097/RTI.0000000000000484.
[16] LIN W, HE C, XIE F, et al. Quantitative CT screening improved lumbar BMD evaluation in older patients compared to dual-energy X-ray absorptiometry[J]. Bmc Geriatrics, 2023, 23(1): 231. DOI: 10.1186/s12877-023-03963-6.
[17] 王盟盟, 张磊, 周凤云, 等. 双层光谱CT、QCT及DXA在骨质疏松诊断中的精确性与效能比较[J]. CT理论与应用研究, 2024, 33(6): 717-724. DOI: 10.15953/j.ctta.2024.086. WANG M M, ZHANG L, ZHOU F Y, et al. Comparative Study of the Accuracies and Efficiencies of Dual-layer Spectral CT, QCT, and DXA for Osteoporosis Diagnosis[J]. CT Theory and Applications, 2024, 33(6): 717-724. DOI: 10.15953/j.ctta.2024.086.
[18] EBBESEN E N, THOMSEN J S, BECK-NIELSEN H, et al. Vertebral bone density evaluated by dual-energy X-ray absorptiometry and quantitative computed tomography in vitro[J]. Bone, 1998, 23(3): 283-290. DOI: 10.1016/s8756-3282(98)00091-x.
[19] WONG M, PAPA A, LANG T, et al. Validation of thoracic quantitative computed tomography as a method to measure bone mineral density[J]. Calcified Tissue International, 2005, 76(1): 7-10. DOI: 10.1007/s00223-004-0020-5.
[20] 张羽, 张宗军, 刘许慧, 等. 胸椎定量CT和腰椎双能X线吸收检测仪对绝经后女性骨质疏松症的诊断差异[J]. 放射学实践, 2022, 37(10): 1205-1210. DOI: 10.13609/j.cnki.1000-0313.2022.10.003. ZHANG Y, ZHANG Z J, LIU X H, et al. Diagnosis difference of thoracic spine quantitative CT and lumbar spine DXA for osteoporosis in postmenopausal women[J]. Radiol Practice, 2022, 37(10): 1205-1210. DOI: 10.13609/j.cnki.1000-0313.2022.10.003.
-
期刊类型引用(1)
1. 张凯杰,丁婷,桂志国,陈平,刘祎,张鹏程,汤豪威. 基于自适应加权增强总变差的CT偏置扫描重建算法. 中国体视学与图像分析. 2024(02): 126-137 .  百度学术
百度学术
其他类型引用(2)



 下载:
下载: